Atopic dermatitis is a challenging, chronic, relapsing inflammatory skin condition that is often accompanied by additional atopic symptoms such as food allergies, allergic rhinitis and asthma.1,2 Atopic dermatitis is assumed to include hereditary as well as environmental factors that precipitate the development and progression of this disease, however the exact aetiology of the disorder is still unclear. Among the immunologic factors contributing to the multifactorial aetiology of atopic dermatitis are alterations related to membrane malfunction, cellular-mediated immune responses and type 1 IgE malfunction.3,4
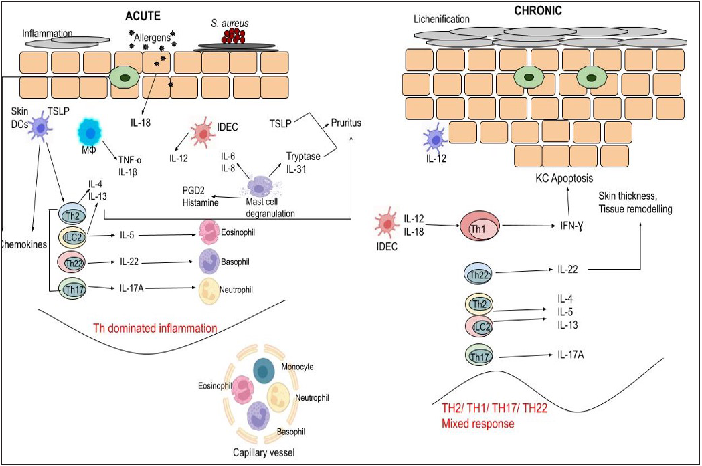
Investigations have demonstrated that skin barrier breakdown and immunological imbalance lead to the development of atopic dermatitis.5 It is primarily a T cell-driven illness since it has elevated levels of cytokines like interleukins and chemokine ligand 18 (CCL18). In addition, there is stimulation of cytokine pathways like Th22, Th17/IL-23 and Th1. DNA methylation is another potential factor that causes atopic dermatitis- [Figure 1].
Export to PPT
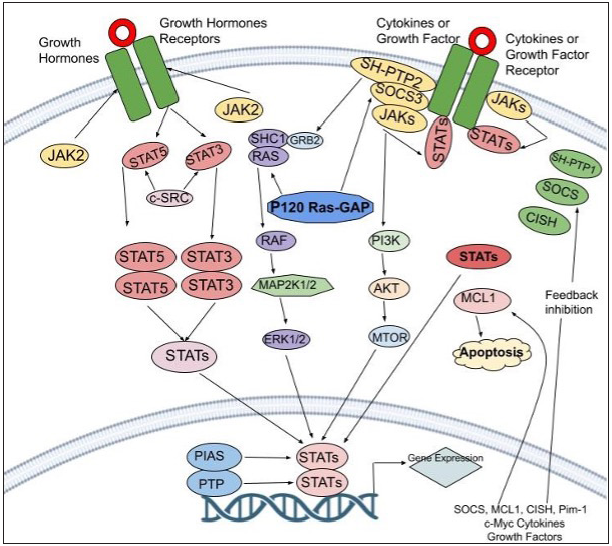
Epidermal abnormalities in individuals with atopic dermatitis were linked to altered gene transcript levels according to an epigenome-wide association analysis in adult atopic dermatitis patients.6 Significant variations in DNA methylation were also found at a total of 19 CpG sites. By demethylating the promoters of the IL-13 and IL-4 genes, GATA3 transcription factor activation in Th2 cells causes the production of IL-4, IL-5 and IL-13 [Figure 2].7
Export to PPT
One of the distinctive features of this phenomenon is the propensity for CD4 cells to develop into cells belonging to the Th2 line. In peripheral blood and tissues, cytokines induce IgE antibodies and eosinophils.8 This inflammation damages the epidermal barrier.
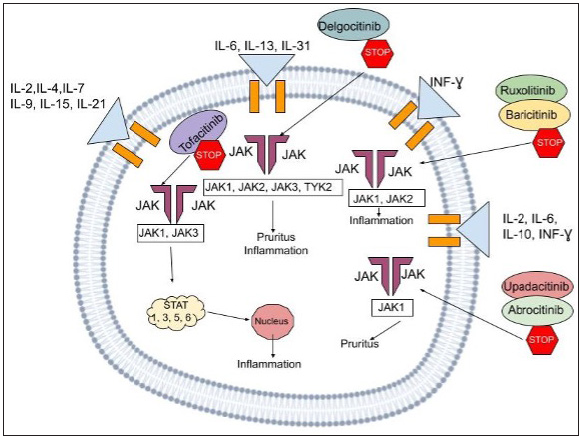
Mechanism of ActionThe JAK-STAT pathway leading to downstream activation triggers the production of several cytokines. Interleukin [IL]-13, IL-4, IL-31 and IL-5 are examples of proinflammatory cytokines that work via this signal transduction channel which serves as a connector between the nucleus and the cell membrane. Mammals have four Janus kinases (JAK1, JAK2, JAK3 and tyrosine kinase 2) and seven STATs (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6) [Figure 3]. When ligands attach to cellular receptors leading to JAK-STAT activation, it stimulates genetic transcription in the nucleus. As a result, Janus kinase suppression may reduce pro-inflammatory cytokine signalling. In atopic dermatitis mouse model studies, Janus kinase inhibitors demonstrated an improvement in skin barrier functioning and a decrease in inflammatory IL levels [Table 1].9,10
Export to PPT
Table 1 Properties of Various JAK inhibitors used in Atopic Dermatitis.
Name Target Formula Route of administration Usage in dermatology Side effects TofacitinibJAK1
JAK3
JAK2
TYK2
C16H20N6O Oral/topicalPsoriasis (oral, topical),
Alopecia areata (oral, topical), Atopic dermatitis (oral, topical),
Vitiligo (oral, topical), Hidradenitis suppurativa (oral) Lichen planus (oral) Dermatomyositis (oral),Cutaneous sarcoidosis (oral),Generalized granuloma annulare (oral),Pyoderma gangrenosum (oral)
Applications site pain and irritation,Upper respiratory tract infections RuxolitinibJAK1
JAK2
C17H18N6 Oral/topical Psoriasis (topical) Vitiligo (oral, topical),Alopecia areata (oral, topical),Atopic dermatitis (topical), Dermatomyositis (oral),Cutaneous lupus (oral),Pyoderma gangrenosum (oral) Applications site pain and irritation Baricitinib JAK1 C16H17N7O2S Oral Psoriasis,Alopecia areata,Atopic dermatitis, Systemic lupus erythematosus Nasopharyngitis and headache Delgocitinib JAK1 C16H18N6O Topical Atopic dermatitis Alopecia areata Nasopharyngitis and application- site skin infections Abrocitinib JAK1 C14H21N5O2S Oral Atopic dermatitis Psoriasis Upper respiratory tract infections, nausea,diarrhea, worsening of atopic dermatitis symptoms and headache Gustacitinib JAK (JAK1, JAK2, JAK 3, TYK2)/SYK C24H28N8O2 Oral Atopic dermatitis Upper respiratory tract infection, headache, and nausea with headache and gastrointestinal symptoms Upadacitinib JAK1 C17H19F3N6O Oral Atopic dermatitis Psoriasis Upper respiratory tract infections, acne and worsening of atopic dermatitis symptoms Topical JAK Inhibitors TofacitinibTofacitinib (TOFA), a JAK1 and JAK3 inhibitor implicated in the Th1 and Th2 signalling pathways, was the first Janus kinase inhibitor to go through substantial human research. A 4-week, phase 2a, double-blind, randomized study involving 69 adults aged 18–60 years found that 2% tofacitinib applied twice daily significantly improved symptoms, leading to a decrease in Eczema area and severity index (EASI) scores of 81.7% as opposed to 29.9% with placebo.11 Nevertheless, as opposed to 9% of control group patients, 17% of the tofacitinib group suffered treatment-emergent side effects, notably nasopharyngitis and upper respiratory tract infections. Another study involving six patients with atopic dermatitis who were resistant to standard therapy found that Scoring Atopic Dermatitis (SCORAD) decreased by 24.3% over 21 weeks (p < 0.05). No notable side effects were reported.12
RuxolitinibExperiments involving transgenic IL-33 mouse models demonstrated the ability of ruxolitinib cream (p < 0.01) in decreasing both thymic stromal lymphopoietin (TSLP) and fluorescein isothiocyanate (FITC)-induced ear swelling (34% and 39%, respectively), abnormal grooming behaviours due to the inflammation and ear punch weight. Further research revealed that it was due to the inhibition of IL-33 and Th-2 proteins. It was coupled with substantial inhibition of the production of interferon signalling pathway mediators, such as IFITM3 (interferon-induced transmembrane protein 3). On histological investigation of tissue samples, ruxolitinib cream significantly reduced the number of mast cells in contrast to vehicle (placebo) cream, providing clear evidence for the drug’s potent anti-inflammatory action.13
There have been numerous studies to establish the role of ruxolitinib in treating atopic dermatitis. Under the registration number NCT03011892,14 a phase 2 clinical research study enrolling 307 adult atopic dermatitis patients was carried out. An Investigator’s Global Assessment (IGA) score of 2 or 3 (mild or moderate) and 3–20% affected body surface area were some of the inclusion parameters. Subjects were randomly assigned to receive RUX (ruxolitinib) cream, active control or vehicle control for 8 weeks. During the second week, more people using 1.5% RUX twice daily than those using 0.1% triamcinolone cream had reached Clinically relevant improvement (CRI) and by the fourth week, near-maximal improvement had been seen. Within 36 hours of starting therapy, over 42.5% of those in the 1.5% RUX group had a minimal clinically significant difference (MCID) in pruritus (vehicle, 13.6%; P < 0.01). Patients receiving 1.5% RUX cream BID demonstrated an increase in Skindex-16 overall scores of 63.5% and 73.2%, respectively, from baseline at weeks 2 and 8.
Kim et al.,15 in phase II research, administered ruxolitinib cream to 252 patients for 4 weeks at four different dosages (0.15% daily, 0.5% daily, 1.5% daily, or 1.5% twice daily), as opposed to triamcinolone 0.1% cream and placebo. All RUX regimens significantly alleviated the distressing symptoms by week 4 with the 1.5% BID group demonstrating the biggest improvement in EASI (71.6% vs. 15.5%; p < 0.0001). The investigation offered strong proof of ruxolitinib’s effectiveness with no significant side effects associated with on-site treatment.
The topical ruxolitinib evaluation in atopic dermatitis studies involved 631 and 618 patients respectively who were randomly assigned to either apply 0.75% RUX cream, 1.5% RUX cream or vehicle cream on the affected areas twice daily continuously for 8 weeks. The primary endpoint of this study was an improvement in the IGA scores. The results obtained thus were highly favourable for ruxolitinib.
In these two phase III atopic dermatitis trials, the average serum concentrations of RUX were minimal and did not change any clinically important haematological parameters. On applying the RUX cream topically, it was reported that there is a relatively low bioavailability which aids in the controlled delivery of the active medication to atopic dermatitis skin lesions. This also reduces the safety concerns related to the oral administration of Janus kinase inhibitors. The responses seen imply that ruxolitinib might be useful in different clinical scenarios. Not only is it beneficial in a broad atopic dermatitis patient population with different racial characteristics, regardless of their baseline disease condition and severity level, but also offers to be an additional treatment option before contemplation of systemic therapy. Because of its effectiveness and safety, RUX cream may replace other topical non-steroidal therapies as the industry standard.16
DelgocitinibDelgocitinib, a pan-Janus kinase inhibitor, demonstrated promising results in non-clinical testing studies. The topical application of delgocitinib lessened inflammation and promoted the manufacture of terminal differentiation proteins, including filaggrin, to treat skin barrier abnormalities. In an animal model of dermatitis, it also decreased skin inflammation. Overall, atopic dermatitis symptom severity, as measured by the modified EASI (mEASI) and IGA scores, had improved dramatically by week 4 of a phase II study. It is hypothesised that the JAK-STAT pathway reduces IL-31 signalling. It can also be due to the inhibition of neural transmission of itch via JAK pathway suppression. These mechanisms are the most probable contributors to the improvement in pruritus that was seen on day 1.17 Japanese patients with moderate-to-severe atopic dermatitis who were 16 years of age or older participated in the phase II long-term study with an accompanying extension study.18 They received treatment for 4 weeks with either delgocitinib 0.5% ointment or vehicle ointment, according to a 2:1 randomization. A 24-week supply of the delgocitinib 0.5% ointment was provided to patients who fulfilled the criteria for Part 2. The main efficacy outcome was the per cent change in the modified EASI score from baseline. The key effectiveness goal for the delgocitinib group was considerably greater than the vehicle group’s in Part 1 and the improvement continued in Part 2.18
Nakagawa et al.19 evaluated the efficacy and safety of delgocitinib ointment application in patients with moderate-to-severe atopic dermatitis using a variety of scales including modified EASI, EASI, pruritus numerical rating scale (NRS), % BSA impacted by atopic dermatitis and Skindex-16, 32, 33. There were only nine treatment-related adverse events, with one person discontinuing the therapy due to the side effects. Nasopharyngitis, acne, fever and Kaposi varicelliform eruptions were the ones most reported. At the end of the part 1 of the study (a 4-week double-blind period) the least-squares mean percent changes from baseline in the modified EASI were significantly greater in the delgocitinib group than in the vehicle group (-44.3% vs 1.7%, P < .001). The improvement was maintained in part 2 of he study (a 24-week extension period).
These results clearly outline the high safety ceiling for delgocitinib.
Oral JAK Inhibitors AbrocitinibPatients with atopic dermatitis are now being tested in clinical studies with this oral JAK1 inhibitor. In one study20 with 267 patients, those consuming 100 or 200 mg of abrocitinib fared better in terms of the fraction of the total number of patients who saw a significant improvement from the initial baseline values. There were no unanticipated safety problems; the action started immediately and it was well tolerated. Nonetheless, at dosages greater than 10 mg, there was a dose-related platelet drop. Even though the abrocitinib regimen was continued, platelet counts gradually returned to baseline after reaching their lowest point at week 4. Most side effects were mild and they are believed to have minimal impact on the compliance given the considerably superior results.21 This research demonstrates that consistent abrocitinib therapy is the preferred therapeutic option for preventing flares in atopic dermatitis cases.22 The phase III JADE MONO-1 investigation, which involved adults and children with atopic dermatitis, and the JADE MONO-2 study detailed the side effects of using the drug abrocitinib. The most visualized negative consequences were nausea, nasopharyngitis, headache and related upper respiratory tract infections.
One unexpected cardiac death was noted in the 100 mg abrocitinib group; however, it was subsequently determined that this death was unrelated to the treatment regimen.
The lipid levels were higher and the platelet counts were lower in JADE MONO-1 and JADE MONO-2, respectively. Unknown mechanisms underlie how abrocitinib therapy alters the platelet count. By week 4, platelet counts decreased after which they became normal. It could be a therapeutic impact of abrocitinib, perhaps exerted via blocking Ashwell-Morrell receptors. It could also be due to the suppression of JAK1 and subsequent downstream effects on platelet or thrombopoietin formation.23 JADE MONO-2 trial had more Asian than European patients. The latter had diverse subsets of helper T cell-fuelled inflammatory activity,24 which could potentially avoid the adverse effects experienced by the patients. The combined findings from the two studies confirm the effectiveness of abrocitinib in a variety of patients, probably because of the suppression of several cytokines connected to atopic dermatitis.
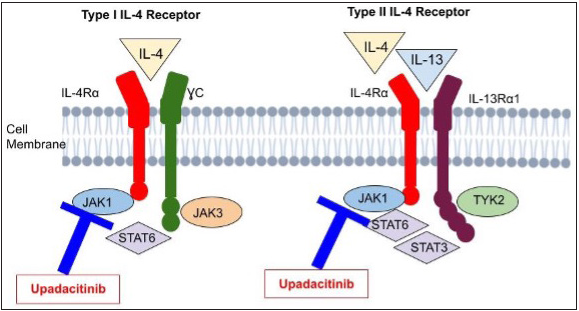
UpadacitinibOriginally envisioned for oral use, this second-generation JAK1 inhibitor was developed to treat rheumatoid arthritis. It selectively suppresses JAK1 by a factor of 74 over JAK3, JAK2 and tyrosine kinase 2 [Figure 4].25 At a dose of 30 mg QD, upadacitinib showed the greatest EASI decrease and seemed to have the optimal benefit–risk ratio. At week 16, all upadacitinib dosages produced statistically significant responses with EASI improvement of 50% or more (EASI50), 75% or more (EASI75) or 90% or more (EASI90) with 50% of persons in the 30-mg group achieving EASI90 in one trial.25 The greatest efficacies for achievement of EASI50 and EASI75 were noticed at week 4 and remained constant until week 16.26 which all dosage? substantially outperformed the placebo in terms of the IGA response and patient assessments of pruritus (improvement in the numerical rating scale). Upadacitinib reduced SCORAD scores when compared to placebo at weeks 8 and 16 with most comparisons showing statistically significant results. The most often reported adverse events were acne, worsening of atopic dermatitis and upper respiratory tract infection; there was no correlation between the dosage and the frequency of any adverse effect. The FDA has designated upadacitinib as a promising systemic therapy for the treatment of patients with moderate-to-severe atopic dermatitis. Another investigation in the phase III stage is currently underway.27
Export to PPT
BaricitinibA JAK1/2 inhibitor used to treat rheumatoid arthritis, baricitinib was recently approved for adults suffering from moderate to severe atopic dermatitis at 4 and 2 mg QID in Europe and Japan.
In early 2019, Guttman-Yassky et al. conducted a promising atopic dermatitis therapy trial using this drug. In phase II randomized, placebo-controlled, 16-week study, baricitinib plus topical corticosteroids were extremely efficacious and safe for adult patients. In this study, 124 patients with moderate-to-severe AD applied topical corticosteroids for 4 weeks before randomization to once-daily placebo QD or 2 mg or 4 mg of baricitinib (BARI). Sixty-one per cent of patients on the 4 mg regimen met the main goal of at least EASI improvement of 50% as opposed to only 37% in the placebo group. Baricitinib significantly improved secondary outcomes, including itch, sleep and quality of life. Mild treatment emergent adverse events were nasopharyngitis and elevated blood creatinine phosphokinase.28
Both the dosage strengths of baricitinib fulfilled the primary outcome of improvement in vIGA (validated IGA) in BREEZE-AD1 and AD2, two parallel, 16-week, phase III, non-U.S. studies. BARI 4 mg met major secondary goals in both experiments. Treatment emergent adverse events frequencies were equivalent and dose independent. BARI caused raised creatine phosphokinase levels in the blood and mild headaches lasting for around 1 day, but not nasopharyngitis or URTI.28
BREEZE-AD5 was a North American–based, 16-week, phase III, randomised double blind placebo controlled trial that pitted BARI monotherapy at 1 mg or 2 mg doses against a placebo in 440 18-year-olds with moderate-to-severe atopic dermatitis. It was found that only BARI 2 mg achieved the primary endpoint (EASI improvement of 75% or more at week 16). The 2 mg baricitinib dose fulfilled practically all secondary objectives of week 16 including vIGA-AD (Validated IGA of atopic dermatitis) of 0 or 1 and itch NRS (Numeric Rating Scale) improvement of 4 or more. Nasopharyngitis, diarrhoea, nausea and URTI were the most common treatment emergent adverse events. Herpes simplex was more frequent with the baricitinib doses (1.4% vs. 2.0% vs. only 0.7% in the placebo group).29
GusacitinibEvidence suggests that gusacitinib inhibits the activity of both Janus kinase and sonic hedgehog kinases. Thirty-six patients suffering from moderate to severe atopic dermatitis participated in a phase Ib study. These participants were randomized to either consume gusacitinib or a placebo in a ratio of 3:1. Three dosage groups – 20 mg, 40 mg and 80 mg – were evaluated for over 28 days.30 The fraction of patients who achieved EASI improvement of 50% or more (20 mg group 20%, 40 mg group 100%, 80 mg group 83%, and placebo group 22%) and EASI improvement of 75% or more (20 mg group 0%, 40 mg group 71%, 80 mg group 33%, and placebo group 22%) was greater for gusacitinib versus placebo. The mild negative side effects that affected patients among different groups were comparable. The study also demonstrated that many blood biomarkers related to Th17, Th22 and Th1 immunity, as well as SELE (the biomarker E selectin) which is associated with atherosclerosis were successfully and significantly attenuated by gusacitinib. In addition, the research demonstrated limited inter-patient variability.
ConclusionVarious studies point to the fact that JAK inhibitors have progressed by leaps and bounds in not only creating and maintaining a disease-free state for atopic dermatitis patients but also in becoming an effective alternative to biologics. There are many advantages of using JAK inhibitors over conventional immunomodulators like precise targeting, long-term safety data, regulation of inflammatory cascade specific for atopic dermatitis, steroid-sparing potential, personalized treatment plans, and potential for combination therapy.
JAK inhibitors also have certain advantages over biologics, including, availability in oral formulations, the ability to target multiple cytokine signaling pathways, a lower cost of therapy, the potential for topical application, fewer immunogenicity concerns, efficacy and safety in pediatric populations, and greater availability.
However, it’s important to acknowledge that, like any treatment, JAK inhibitors do carry potential side effects that require careful consideration. Available evidence suggests they may be useful in AD care, giving patients hope for effective and accessible medication.
留言 (0)