In patients who require prolonged nutritional support, percutaneous gastrostomy tubes (PEG) are invaluable medical devices for obtaining enteral access, particularly in patients with dysphagia secondary to neurological disease.[1,2] However, a major disadvantage of PEG is its high failure rate due to the inability to transilluminate the abdominal wall, which can drastically reduce the precision of PEG placement given anatomical variance among patients.[3] In situations where greater localization of tube placement is required, radiologically inserted gastrostomy (RIG) has become a worthwhile access modality. RIG is a minimally invasive technique that uses fluoroscopy to guide the placement of a PEG tube. Notably, RIG offers an advantage over PEG as it eliminates the need for an endoscope, which in turn preserves the airway for other critical uses such as concomitant intraoperative non-invasive ventilation (NIV). This noteworthy advantage along with real-time anatomical detail in PEG tube placement is beneficial in patients suffering from neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS).[2] However, studies have pointed to greater gastrostomy failure rate and postoperative aspiration in PEG placement for ALS patients.[4] Our study assessed how RIG affected overall mortality rate, 30-day mortality rate, and major and minor post-operative complication rates in patients with neurological disease necessitating enteral support: An area of research where there is a paucity of available data in current literature. In addition, we provide a comparison of how two types of gastrostomy tubes (balloon-assisted gastrostomy [BAG] vs. non-balloon-assisted gastrostomy or dilator) used in RIG can affect performance with regard to pertinent radiologic parameters as well as patient pain management.
MATERIAL AND METHODS Data collectionA single-center retrospective review of all RIG tube placements from July 2017 to September 2020 was performed at a tertiary care hospital. 152 patients were included in this study, with 104 patients and 48 patients in the BAG and dilator groups, respectively. Patients included in the study were diagnosed with neurological diseases as clinically determined by a board-certified neurologist. Within this category, our study also included three indications for neuromuscular disorders which were ALS, myasthenia gravis, and muscular dystrophy. This retrospective study, which was performed under clinical study guidelines, was approved by the Institutional Review Board (IRB) and determined to be IRB exempt. The demographic information and radiation-related data were collected based on electronic medical records. Fluoroscopy time, peak radiation dose, pain management, and post-operative complications (classified as CIRSE Grades 1 and 2) for each procedure were reviewed to evaluate for statistical differences.[5]
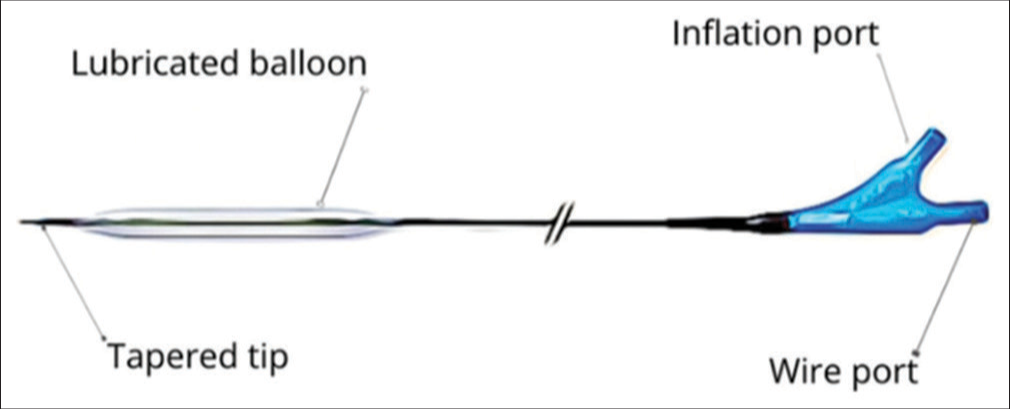
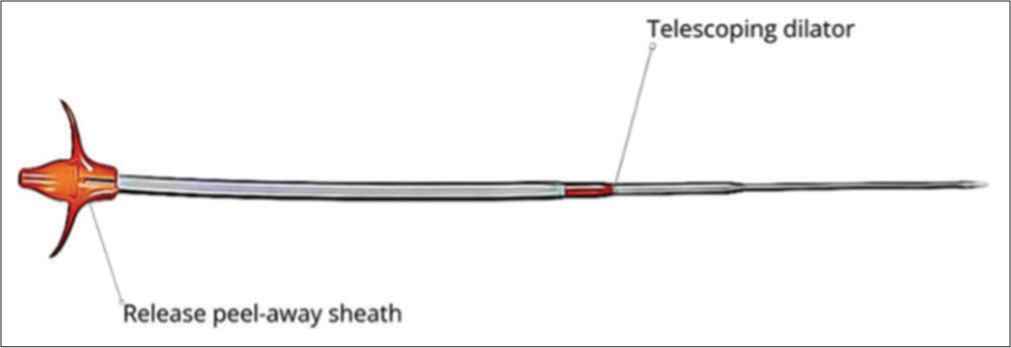
RIG tube insertionRIG tube insertion is a minimally invasive procedure is done under local anesthesia and conscious sedation. After preparing the abdominal region with sterile techniques and administering midazolam and fentanyl citrate for sedation and pain relief, a pre-procedural ultrasound is obtained at the left edge of the liver to locate the epigastric arteries to reduce intraoperative bleeding. The gastric lumen is gently insufflated with air through an existing nasogastric tube, and a gastropexy procedure is performed using three T-bar fasteners arranged in a triangular fashion. While using fluoroscopic guidance, if rapid decompression of the stomach due to peristalsis is noted, 1 mg of glucagon IV may be given to reduce peristaltic forces and maintain optimal insufflation. Next, a small incision is made at the center of the gastropexy fasteners to access the stomach, and a guidewire is advanced under fluoroscopic guidance through the abdominal wall. The BAG approach uses a Mustang balloon for tract dilation, whereas the dilator group employs a telescoping dilator system with an introducer sheath. Once the tract is dilated, a gastrostomy tube is inserted and secured in place. When using RIG insertion, typically a 6 mm × 40 mm or 7 mm × 40 mm Mustang balloon is used to secure a 16-French gastrostomy catheter. The correct placement is confirmed by encountering resistance during catheter retraction. For both approaches, the retention balloon is filled with sterile water to establish stability, and the catheter is retained securely using gastropexy sutures. In case of discomfort, the retention sutures can be selectively released while maintaining catheter stability through the balloon. The selection of gastrostomy tube technique, BAG [Figure 1] or dilator [Figure 2], was based on the operator’s preference. Each procedure was carried out by seven experienced interventional radiologists, all of whom had served as faculty members ranging from 2 to over 20 years. Crucially, each operator displayed equal proficiency in both balloon and dilator percutaneous gastrostomy procedures, ensuring consistency and eliminating potential bias arising from differences in training.
Export to PPT
Export to PPT
Post-procedure dischargeData were collected on the pertinent radiological variables, technical outcomes, procedural issues, and post-operative catheter-related complications. Repeat evaluation of the access site was performed for all patients before discharge. The follow-up appointment was made based on a future management plan.
Statistical analysisTo evaluate the differences, the data regarding fluoroscopy time (min), radiation dose (mGy peak skin dose), procedure time (min), pain management (versed in mg and fentanyl in mcg), and post-operative complications (major and minor) were collected for both groups. The outcomes of the treatment groups were analyzed using the Chi-square analysis. A multivariate analysis of the following variables (gastrostomy tube type, body mass index [BMI], age, and sex) was conducted to assess each individually as a prognostic indicator on complication rates P < 0.05 were considered statistically significant. All results of this study were analyzed using appropriate statistical software (SPSS Statistics 26.0, IBM Inc.).
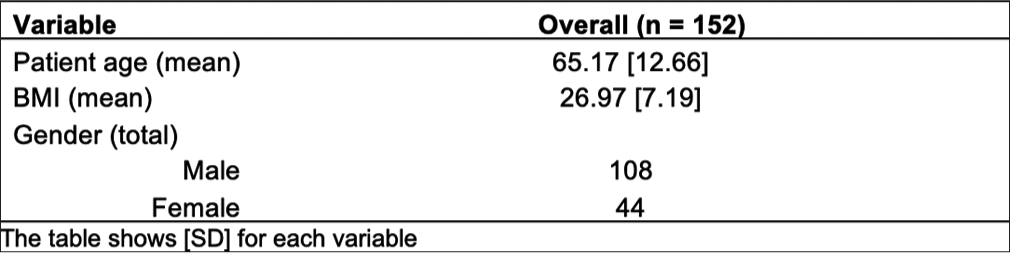
RESULTSIn this study, there were 152 patients with a mean age of 65.17 years (interquartile range [IQR] = 12.66) [Figure 3]. The average BMI in our population was 26.97 (IQR = 7.19) [Figure 3]. Male was the predominant gender (71.1%) [Figure 3].
Export to PPT
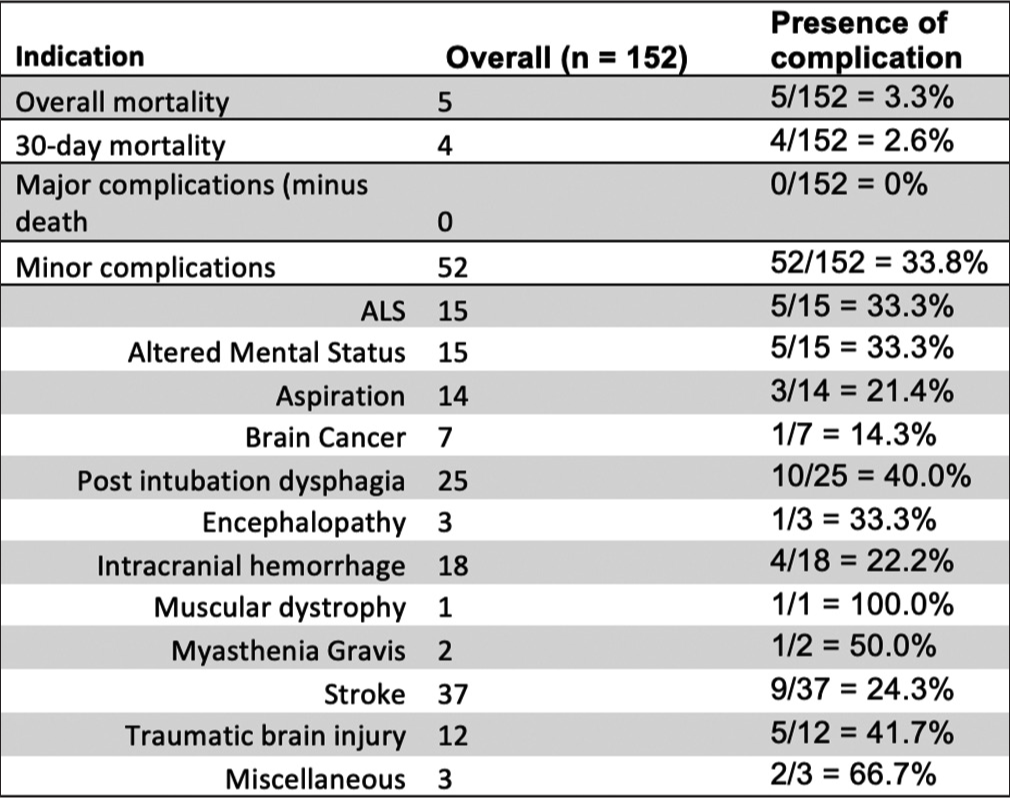
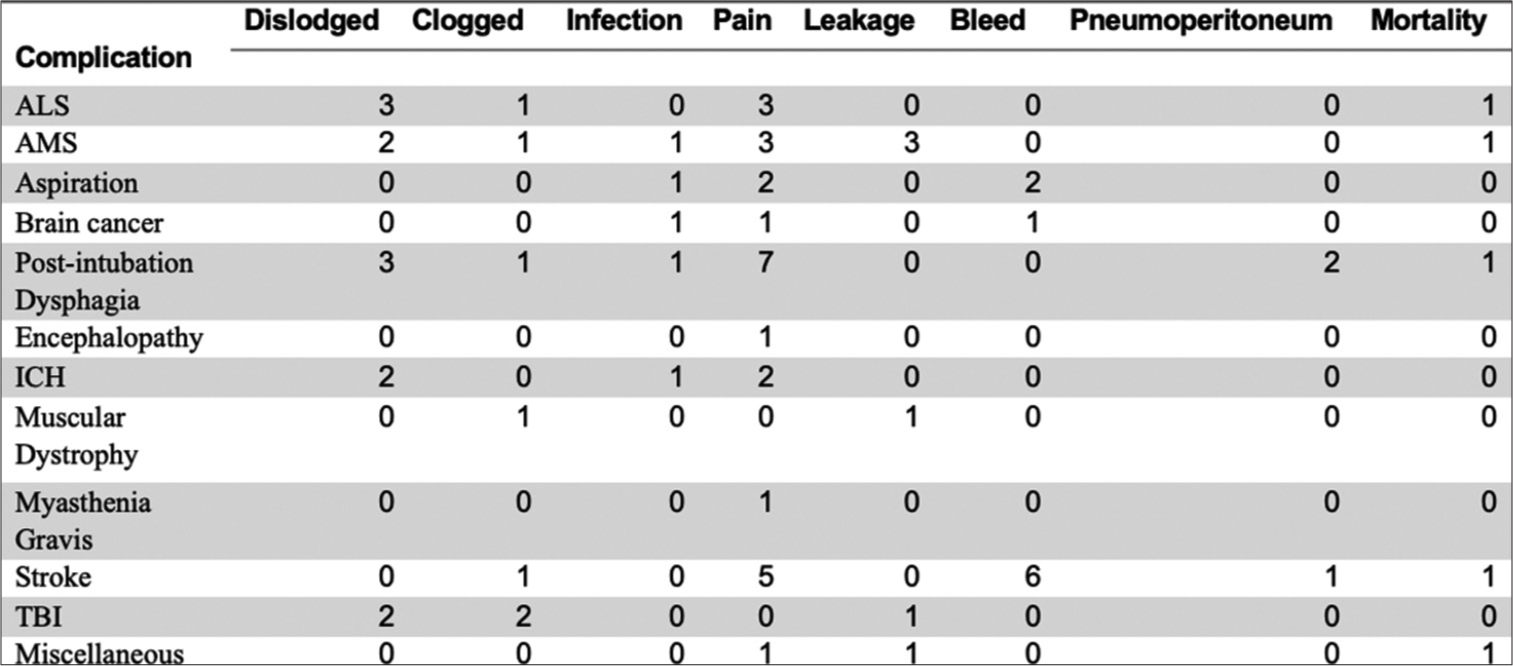
Our study’s overall complication rate was 33.8%, overall mortality rate was 3.3%, and 30-day mortality rate was 2.6%. No additional major complications were reported as defined by CIRSE classification.[5] The most common indication was stroke (24.3%), followed by post-intubation dysphagia (16.4%) and intracranial hemorrhage (11.8%). ALS and altered mental status showed similar prevalence at 9.9% [Figure 4]. Other indications included aspiration (9.2%), traumatic brain injury (TBI) (7.9%), brain cancer (4.6%), encephalopathy (2%), myasthenia gravis (1.3%), and muscular dystrophy (0.7%) [Figure 4]. Miscellaneous (2.0%) included medication-induced toxicity and obstructive hydrocephalus. When comparing within each subpopulation, we note patients with neuromuscular disorders showcasing similar minor complication rates such as ALS with 5/15 patients (33.3%), myasthenia gravis with 1/2 patients (50%), and muscular dystrophy with 1/1 patients (100%) [Figure 4].
Export to PPT
Dislodgment (3/15) and pain (3/15) were common minor complications in ALS patient population [Figure 5]. There was one reported death in ALS population [Figure 5]. There was one instance of leakage in a muscular dystrophy patient and pain in myasthenia gravis patient [Figure 5]. AMS patient population had equal incidences for pain and leakage (3/15), followed closely by dislodgment (2/15), clogged tube (1/15), and infection (1/15) [Figure 5]. Brain cancer patients experienced similar complication rates such as infection, pain, and bleeding. Most common complication in post-intubation dysphagia group was pain (7/25), followed by dislodgment (3/25) and pneumoperitoneum (2/25) [Figure 5]. Stroke patient population had the highest incidence of bleed (6/37) and pain (5/37) [Figure 5]. TBI had equal complication rates of dislodgment and clogged tube (2/12), and one instance of tube leakage. Miscellaneous group experienced equal rates of pain (1/3) and leakage (1/3) with one reported death [Figure 5].
Export to PPT
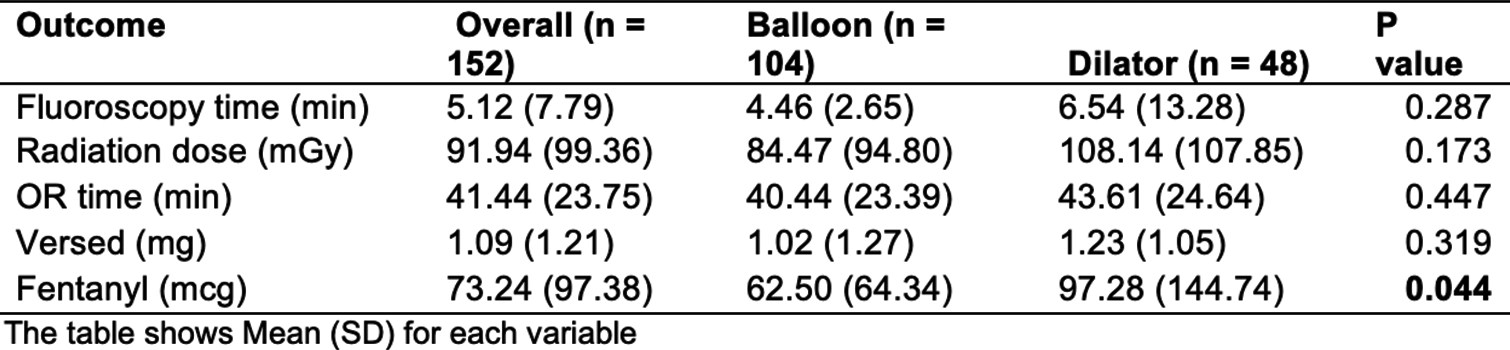
There were 104 patients in the balloon group and 48 patients in the dilator group [Figure 6]. Fluoroscopy time was shorter in the balloon group compared to the dilator group, though not statistically significant (Avg = 4.46 min vs. 6.54 min, P = 0.287) [Figure 6]. Similarly, the balloon group trended toward a lower radiation exposure dose (mGy PSD), though not statistically significant (Avg = 84.47 mGy vs. 108.14 mGy, P < 0.173) [Figure 6]. Regarding procedural details, the balloon group required lower operating time (40.4 min vs. 43.61 min, P < 0.447). Balloon group received significantly lower preoperative sedation in the form of fentanyl (62.5 mcg vs. 97.3 mcg, P < 0.05) [Figure 6]. The use of versed was also lower in the balloon group, although not statistically significant (1.02 mg vs. 1.23 mg, P < 0.319) [Figure 6].
Export to PPT
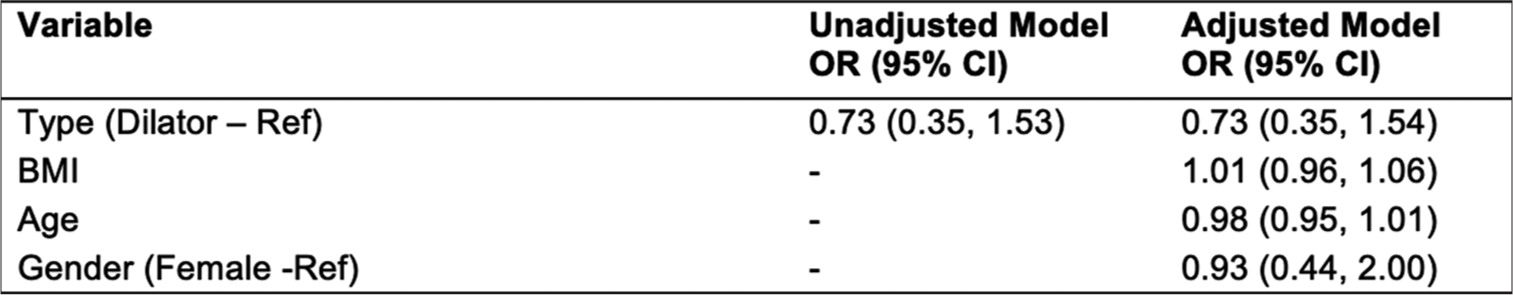
In a logistic regression model, dilator showed no statistical difference compared to BAG toward complication rates (OR: 0.73, 95% confidence interval [0.35, 1.54]) [Figure 7]. BMI, age, and gender did not significantly affect minor complication rates [Figure 7].
Export to PPT
DISCUSSIONPEG tube placement is a commonly used method for enteral nutrition support in patients with neurological disorders.[2] Among the most significantly studied indications for PEG placement include dysphagia secondary to cerebrovascular disease, motor neuron diseases (MND), movement disorders, or dementia.[6] The overall efficacy of gastrostomy tube placement has been shown in literature in patients on nasoenteric support who were stratified with having a moderate-to-severe risk of malnourishment.[6] However, as aforementioned, traditional PEG placement does not always lead to precise placement given the lack of real-time fluoroscopic guidance. In these circumstances, RIG tube insertions are invaluable. In addition, as mentioned before, RIG approach is beneficial in neurodegenerative disorders requiring continuous respiratory support with NIV.[2] Although limited, there is some evidence to support the preferential use of RIG; this includes a notable retrospective study of ALS patients which found greater failed gastrostomy tube placement (15.7% PEG and 1.9% RIG) and greater post-procedure rates of aspiration (10.5% PEG and 0 RIG) in the PEG group.[4] Our investigation expands upon this population in assessing complications and mortality rates following RIG placement in a wide array of neurological diseases.
Although RIG intervention has shown to have several benefits in patients with neurodegenerative disorders, postoperative complications must also be discussed. The overall complication rate (33.8%), in our study, was lower compared to previous studies in PEG placement.[2] Overall mortality rate and 30-day mortality rate were found to be lower than in previous studies.[2,7] In addition, we found that minor complications following RIG were more frequent in patients with rapidly progressive neuromuscular disorders, such as our ALS (5/15, 33.3%), myasthenia gravis (1/2, 50%), and myotonic dystrophy (1/1, 100%) groups when compared to patients with other neurological impairments.[2,8] Although the sample size for ALS (and myasthenia gravis and myotonic dystrophy) was small in our study, this finding is consistent with other studies that have reported higher complication rates in patients with ALS.[9] Typically, patients with ALS (and myasthenia) who require NIV and undergo gastrostomy tube placement exhibit a high frequency of complications, likely due to respiratory compromise affecting the proper functioning of the gastrostomy tube. This is also observed in our study with high incidences of dislodgment, pain, and clogging in this subset of patients. The findings of our current investigation further underscore the significance of the Allen et al.’s study which shows markedly improved outcomes for ALS patients assigned to the RIG group.[4]
Complication frequency in stroke patients (9/37, 24.3%) was comparable to data from other studies.[10] A large percentage of our patient population had indications for stroke-related dysphagia (37/152, 24.3%) and dysphagia which was attributed to a multifactorial cause, unrelated to stroke (25/152, 16.4%). The authors would like to mention that multifactorial dysphagia received its own category based on dysphagia alone being considered an impairment from baseline neurological functioning. Several minor complications were recorded within our study, such as local wound infection, pain at insertion site, peristomal leakage, tube dislodgment, and pneumoperitoneum.
For RIG placement, our patient population was subdivided into balloon versus dilator gastrostomy placement. This division allowed us to examine the outcomes and variables associated with each technique. Multivariate analysis showed no significant difference between balloons or dilators on minor complication rates, suggesting both dilators and balloons can be used effectively for RIG tube insertion. We found that the use of balloons during RIG placement was associated with lower pre-operative fentanyl requirements, whereas other radiological variables were found to be statistically insignificant. Our study showed a decreased odds ratio of minor complications with dilator use, though it did not reach statistical significance. It is important to note that the interpretation of these results was confounded by other variables, such as operator technique and patient comorbidities, which may have influenced the outcomes.
In addition to operative-related complications, patient response to gastrostomy placement may be predicted by several factors including age, BMI, stratification of disease severity, and the potential role for serum markers of inflammation and nourishment status. Factors such as BMI, age, and gender did not show correlation within our study. Previous studies found that older age, BMI <20 kg/m2, and presence of decubitus ulcers were significantly predictive of mortality.[11] Although declining health status plays a role in outcome, multiple serum markers, such as albumin and CRP, have been analyzed in recent years to aid in the justification of PEG/RIG insertion.
It would be remiss to not discuss some of the reported drawbacks of a RIG technique. A prospective cohort study showed that RIG was associated with greater adverse events (22% PEG vs. 51% RIG), though these mainly included local and self-limiting events such as stromal reaction.[12] Although RIG should theoretically improve rates of wound reaction, since the tube will not be contaminated by oral microbiota as seen in PEG, this study postulated that delayed removal of anchor sutures contributed to the observed results.[12] In addition, RIG has been associated with greater rates of tube dislodgment than PEG, and Sundbom et al. suggest that this may be due to differences in tube size (20 Fr in PEG vs. 18 Fr in RIG) and the use of balloon to secure RIG versus flat dome to secure PEG.[12] This was reflected in our study, in which tube dislodgment was frequently reported. Tube migration may be prevented by adequate manipulation of the external bumper to lie 1–2 cm above the skin.[6] Further studies should be carried out comparing the perioperative benefits, risks, and complications of these two procedure styles to better guide management in this select group of patients.
However, a feared major complication of stroke is aspiration pneumonia; often, PEG fails to reduce this risk with as many as 18% of stroke-related dysphagia patients suffering from aspiration pneumonia.[6] It is critical to mention our altered mental status patient population since most advanced AMS patients suffer from severe effects of feeding difficulty due to combined factors such as altered sense of smell, lack of interest, apraxia of eating, and an inability to protect their airway, which altogether drastically increases their risk for aspiration pneumonia.[6] For these patients who are at the highest risk of mortality and morbidity from aspiration, PEG remains a controversial topic.[2] A retrospective analysis found that PEG placement failed to improve hospital readmission rates, overall survival at 1 month, and mortality rate within 1 year in patients with cognitive impairment when compared to stroke and MND patients.[13] Special precautions should be endorsed perioperatively to reduce the risk of minor and major complications such as ensuring standard procedural sterility techniques, adequate postoperative tube maintenance, reducing the volume of feeds, and positioning with elevation of the head of the bed.[6] Again, further studies should investigate and compare the rates of major complications such as aspiration pneumonia in PEG versus RIG for patients suffering from a wide array of neurological diseases.
The present study has limitations. First, this was a retrospective analysis without a control group (i.e., traditional PEG cohort). Second, because of a small sample size, the reported prognostic factors may not be transferable to a large patient cohort. Large, randomized, controlled trials could provide more definitive evidence for how a wide spectrum of neurological diseases respond to gastric tube placement. The lack of availability of robust studies has limited the comparison of efficacy between PEG and RIG. Among the few cohort studies that have been conducted, there is a notable divide between advocacy for PEG and RIG.[12]
Finally, the authors wish to mention that a strong clinical indication for RIG must often be weighed against patient autonomy, overall prognosis, ethical considerations, and anticipated quality of life post-procedure.[2] Care burden should also be considered this may be the beginning of palliation for most patients suffering from advanced neurological disorders. Procedure costs dictated by the 2023 Medicare National Average Payments recent data show the initial placement of a gastrostomy tube for a hospital outpatient costs $1,742. Per this report, the cost remains the same for the initial operative placement of the tube when either endoscopic or fluoroscopic guidance is used (including contrast injection). Costs begin to vary when fluoroscopic guidance is required for gastrostomy tube replacement.[14] Although indication for RIG may be clear in some patients with end-stage diagnoses, the severity and progression of the disease ultimately affect the clinical response to RIG and willingness to undergo the procedure.[15] Of utmost importance is to consider the risks versus benefits for this vulnerable population who experience the greatest risk of morbidity and mortality from their primary neurologic condition and potential other comorbid illnesses.
CONCLUSIONThis study examines the efficacy and safety of RIG tube placement (RIG) in patients with significant neurological-related impairments. RIG interventions can offer advantages, especially in patients with ALS and myasthenia gravis patients, allowing for continuous respiratory support with NIV during the procedure. The overall complication and mortality rate indicate a favorable safety profile for RIG. Our study found similar complication rates between BAG and dilator techniques, suggesting both can be used effectively, Overall, RIG remains a valuable modality in providing enteral support in a wide array of neurological disorders, providing opportunities for enhanced patient care and quality of life post-procedure.
Comments (0)