Granulomatous prostatitis (GP) is a benign inflammatory condition of the prostate, characterized by presence of granulomas.[1,2] It is a rare form of chronic prostatitis with incidences reported in literature ranging from 0.65% to 1.5% incidence of all inflammatory prostate lesions.[3-7] The etiology of GP is unclear; most cases are idiopathic. The leading hypothesis is the immune response to extra-ductal prostatic secretions, refluxed urine, bacteria, and colloidal contents trigger this cascade of events.[7,8] Existing data suggest increased incidence of GP in cases with recurrent urinary tract infections, prostate interventions including radical proctectomy to core needle biopsy, and instillation of bacillus Calmette–Guérin (BCG) into the bladder.[3,9-11] GP can occur in normal or hyperplastic gland, or in carcinomatous prostate.[3,10]
On gross pathology, the prostate is firm to stony hard and cut sections show architectural distortion with formation of yellow granular nodules. Large nodular aggregates of histiocytes, epithelioid cells, multinucleated giant cells, and plasma cells are present on histopathology.[12]
Radiological features of GP overlap with those of prostate adenocarcinoma which can make diagnosis by imaging alone difficult. Lee et al., in a retrospective study of 16 patients, found that in cases with GP, there is diffuse change involving >50% of the gland, with extracapsular extension and rim-enhancing areas.[13] GP should be considered as a differential diagnosis in the setting of recurrent urinary tract infection or prior BCG treatment.[13] Interestingly, Lee et al., observed lower apparent diffusion coefficient (ADC) values in cases with GP, mean ADC value of 702 ± 79 × 10−6 mm/s2.[13]
While there is overlap of the imaging features with prostate adenocarcinoma, the following magnetic resonance imaging (MRI) characteristics and patterns are described in the literature and can suggest diagnosis of GP in the appropriate clinical setting. By T2-weighted imaging GP can present with diffuse or nodular pattern of low-signal intensity lesions, loss of transitional zone architecture, and in few cases presence of capsular irregularity.[13-16] On diffusion-weighted images (DWI), there is high-signal intensity with corresponding low signal on ADC.[13-16] Post-contrast imaging shows early diffuse enhancement with or without rim enhancement from caseous necrosis and abscess formation.[13-16] These imaging features are suggestive of the diagnosis; however, biopsy is typically necessary for confirmatory diagnosis.
Here, we present a case of GP in the setting of BCG therapy for bladder cancer.
CASE REPORT Clinical presentationThe patient is a 70-year-old male with medical history of low-grade bladder cancer (non-invasive low-grade papillary urothelial carcinoma) treated with intravesical BCG therapy (#12 cycles), last cycle administered 12 years before current presentation. On a regular follow-up visit, his prostate-specific agent (PSA) was elevated to 6.4 ng/mL. The patient denies a family history of prostate or urothelial cancer. The patient is a former smoker with eight pack years with smoking cessation following the diagnosis of bladder cancer.
ImagingMultiparametric prostate MRI (mpMRI) with and without contrast was performed, which showed multifocal lesions in the peripheral zone, the largest lesion in right base measured 14 mm in largest dimension, and most compatible with prostate imaging–reporting and data system (PI-RADS) 4 (High-clinically significant prostate cancer is likely to be present).[17-20] The smaller bilateral lesions (≤5 mm) demonstrate imaging features most compatible with a PIRADS 3 (Intermediate – the presence of clinically significant prostate cancer is equivocal) [Figures 1-3]. The lesions were assigned PI-RADS scores; however, GP was offered as an alternative diagnosis given the prior history of BCG therapy and multifocal, nodular distribution. Targeted tissue sampling was recommended.
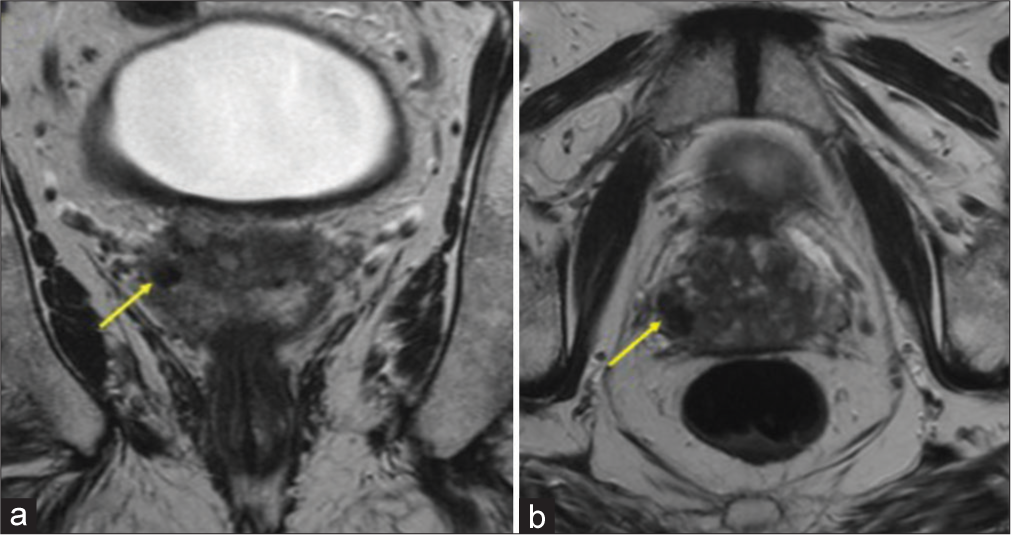
Export to PPT
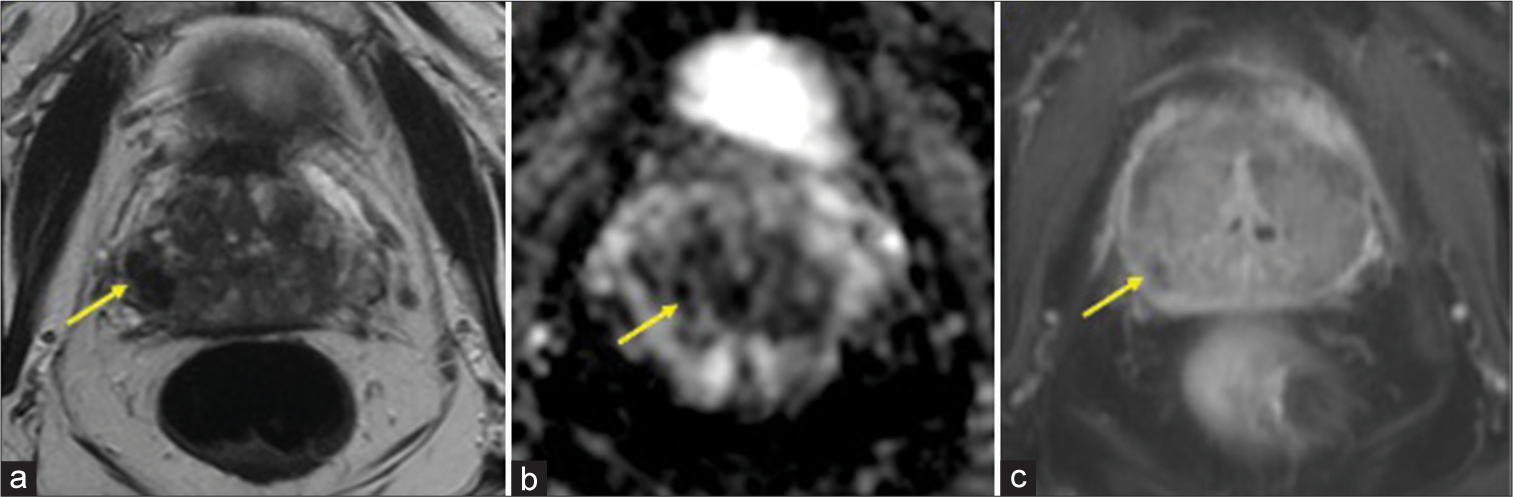
Export to PPT
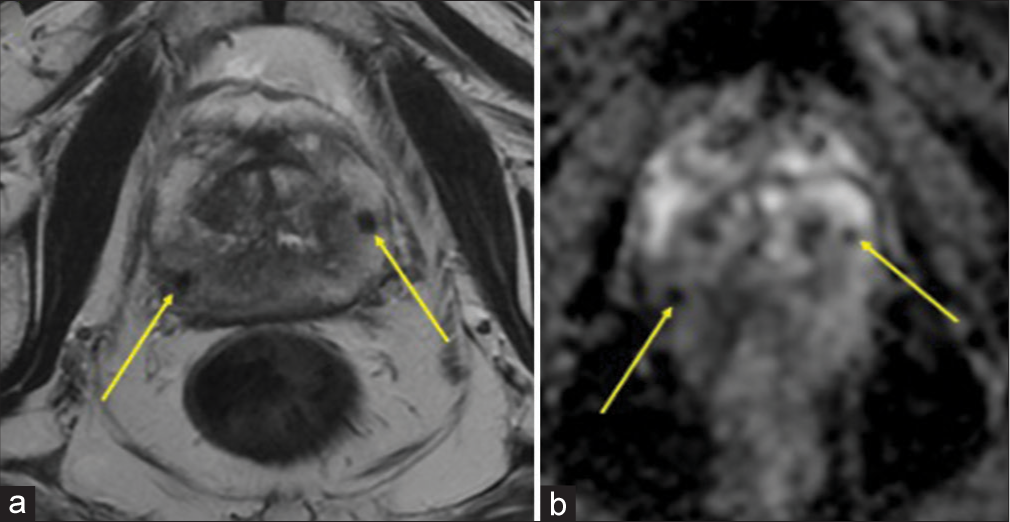
Export to PPT
On transrectal ultrasound, the prostate measured 41.7 mm × 48.9 mm × 27.7 mm (length, width, and height) with a calculated volume of 29.5 cubic cm. No hyperechoic or hypoechoic areas were visualized within.
BiopsySix random biopsies were obtained from the right and left portions of anteromedial, anterolateral, posteromedial, posterolateral, medial base, and lateral base of the prostate gland. Three additional biopsies were taken from the MRI-targeted lesions in the right base, left mid, and right anterior lobe. PSA density was 0.22. Patient tolerated the procedure without any complications. Targeted biopsy of the MRI lesion from right base, left mid, and right anterior lobe was reported as chronic prostatitis with granulomas, with atrophy and fibrosis, compatible with GP [Figure 4]. All submitted biopsy samples were negative for prostatic adenocarcinoma. In addition, random biopsies from different areas also revealed evidence of chronic prostatitis with granulomas and atrophy.
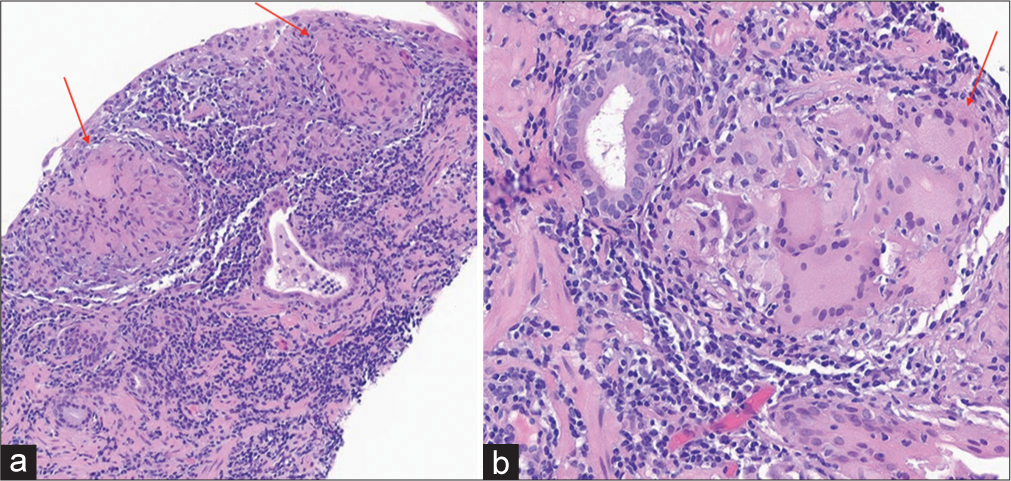
Export to PPT
DISCUSSIONPI-RADS was developed by an internationally representative group involving the American College of Radiology, European Society of Urogenital Radiology, and AdMeTech Foundation. It provides a structured reporting scheme for mpMRI in the evaluation of suspected prostate cancer in treatment of naive prostate glands.[17] The use of PI-RADS does not contribute significantly to differentiate GP from malignancy. At most institutions, a PI-RADS score of 3 or greater prompts further evaluation with biopsy.
Symptomatically, patients present with fever, chills, urgency, frequency, and dysuria or maybe asymptomatic.[21] Clinically, patients with GP may present with an indurated prostate or a non-tender, palpable nodule adjacent to the prostate on digital rectal examination.[22,23] A history of intravesical BCG-therapy or recent urinary tract infection can indicate the need for imaging with a suspicion of GP. Clinical GP may present with normal to elevated serum PSA levels.[24] Increased PSA levels in the months post-BCG instillation are a frequent finding.[25] Although PSA is a screening marker found to be elevated in patients with prostatic carcinoma, it is non-specific and, further, evaluation is needed to differentiate the cause.[10,24]
Pathologic featuresThe pathogenic mechanisms of GP are thought to be either direct infection or inflammation.[26] Intraprostatic reflux of BCG-contaminated urine from the bladder establishes the prostatic nidus and initiates the chronic inflammatory response, resulting in the formation of non-caseating or caseating granulomas within the prostate or periprostatic tissue, including the prostatic urethra.[27] Several micro-organisms including tuberculosis, syphilis, brucellosis, viral, and fungal entities can cause GP. However, the infectious cause of BCG-induced GP is Mycobacterium tuberculosis.
Prostatic adenocarcinoma, non-specific GP, post-biopsy hemorrhage, benign prostatic hyperplasia nodules, and acute and chronic prostatitis can all mimic BCG-related GP.[24] Rarely, adenocarcinoma may coexist with GP.[26] Asymptomatic individuals do not require any specific treatment as GP is self-limited but tuberculostatic drugs may be initiated and systemic disease warrants the addition of anti-inflammatory drugs like prednisone.[26]
Histologically, epithelioid cell granulomas with Langhans giant cells may be seen. A prominence of epithelioid histiocytes or the appearance of a xanthogranulomatous pattern may require a follow-up immunohistochemical examination since this mimics adenocarcinoma. In cases of GP, immunohistochemistry reveals antibodies to CD68 (histiocyte marker) and in prostatic cancer, antibodies to cytokeratin 5/6 (basal cells) or cytokeratin AE1/AE3 (epithelial cells).[9] Acid-fast bacteria (AFB) staining may reveal M. tuberculosis or may be negative. Other special stains commonly do not detect any microorganisms.
Radiologic featuresMultiparametric MRI has become a commonly utilized imaging tool to assess the presence of clinically significant prostate cancer (Gleason 7 or greater). The typical mpMRI findings of GP are: (i) low-intensity signal on T2-weighted images, (ii) hyperintense signal on DWI, (iii) low-signal/values on ADC maps, and (iv) peripheral rim enhancement on contrast.[22,28,29]
A way to discern the diagnoses was proposed by Lee et al. by describing three different mpMRI patterns: A, B, or C.[13] Peripheral zone lesions with diffusion restriction and moderate to marked homogeneous enhancement on dynamic contrast-enhanced images were classified as type A. This pattern is indicative of highly cellular non-necrotic granulomas. The lesions with diffusion restriction and a poorly enhancing component were classified as type B. These are seen in necrotic GP with the central necrosis having a high-signal density on T2-weighted images and focal hyperintensity on high b-value images. A low-signal intensity on high b-value DWI (b = 1000 s/mm2) is considered characteristic of type C. The suggested protocol is to use these findings alongside other diagnostic modalities and sequential monitoring on follow-up.[27,30]
Another study identified three main patterns on MRI-diffuse, nodular, and cystic with mural nodule.[31] A notched polygonal prostatic nodule is a diagnostic feature of GP. Sarkis et al. established the set of the prostatic imaging changes seen before and after BCG administration.[32] A comparative study with pre-operative PSA and prostate MRI can help rule out prostate carcinoma.[33] Gottlieb et al. recognized two-phased patterns of GP, acute and chronic, based on the temporal distribution of prostatic changes on mpMRI using the high-b-value sequence.[29]
Transrectal prostatic sonography may demonstrate nodular/diffuse hypoechoic areas in the prostrate, which is a non-specific finding. Ultrasound examination only serves to complement the digital rectal examination and suspicious findings in the peripheral zone of the prostate must be followed by histological examination on biopsy.[21,23,34] Increased focal prostatic uptake on 2-deoxy-2-(fluoro-18F)-D-glucose positron-emission tomography–computed tomography (PET-CT) can be indicative of malignancy but in patients with a high degree of suspicion for GP, it is considered a benign finding.[35,36] PET-CT merely indicates hypermetabolic state of the tissue in GP.[37]
Based on MRI alone, GP and prostate cancer cannot be definitively distinguished since they share common imaging features. Due to their overlapping features, biopsy is necessary for histologic confirmation, although the MRI findings can be suggestive in the setting of prior BCG administration. Therefore, if the biopsy and MRI findings are concordant, the information is critically useful in the event of future MRI examinations to avoid unnecessary re-targeting and re-biopsy of known foci of GP.
CONCLUSIONA combination of clinical evaluation, imaging techniques like mpMRI, and histological examination can help in the accurate distinction between GP and prostate adenocarcinoma. Knowledge of GP and it’s features are important considerations for radiologists interpreting initial and follow-up prostate MRI examinations.
Comments (0)