Spinal epidural hematoma is an uncommon condition typically characterized by sudden, intense pain at the hemorrhage site, which can radiate to the limbs. It may quickly progress to significant neurological impairments. The underlying mechanisms are often unclear, but in the lumbar region, it is commonly attributed to the rupture of Batson’s vertebral venous plexus.[1] Syringomyelia, on the other hand, is a distinct disorder caused by disruptions in normal cerebrospinal fluid (CSF) flow. A thorough imaging assessment using advanced three-dimensional constructive interference in steady-state (CISS) and four-dimensional contrast imaging techniques is essential for identifying the underlying lesion, as this will guide the appropriate surgical approach.[2]
Syringomyelia’s causes include factors that disrupt CSF circulation. Typically, it results from obstructions in the spinal subarachnoid space. The potential causes include:
Syrinx with no identifiable cause
Syringomyelia with obstructions at the foramen magnum (developmental):
Chiari Type 1 Malformation (CM1): Most commonly associated[3]
Basilar invagination.
Syringomyelia with other spinal cord diseases (acquired):
Post-inflammatory:
Postinfectious: Granulomatous infections (e.g., tuberculosis and fungal), postoperative meningitis
Chemical/sterile inflammation: Following subarachnoid hemorrhage or myelography (metrizamide).
Post-traumatic
Spinal Cord Tumors: Especially intramedullary tumors such as hemangioblastoma
Secondary myelomalacia: Including cord compression (due to herniated discs, spondylosis, tumors), infarction, and hematomyelia.[4]
Epidemiological information on syringomyelia is sparse. Studies suggest its prevalence ranges from 8.4/100,000 to 0.9/10,000, with variations across different ethnic and geographic groups.[5]
Magnetic resonance imaging (MRI), with or without contrast, is the preferred diagnostic tool. It provides detailed anatomical information and allows for accurate visualization of the syrinx in both sagittal and axial planes. MRI effectively shows the location, size, and extent of the syrinx cavity and the degree of cerebellar tonsillar ectopia. In patients with CM1, MRI typically shows compression of retro-cerebellar CSF spaces. It also helps exclude cystic lesions or spinal tumors. Leptomeningeal enhancement may indicate infection, while MRI can reveal arachnoid scarring. In addition, MRI can monitor syrinx progression over time, documenting the natural history of syringomyelia. This imaging method also enables non-invasive analysis of CSF hydrodynamics, including disturbances in CSF velocity/flow at the foramen magnum (particularly in patients with less than 5 mm tonsillar ectopia), spinal cord wall motion, and syrinx fluid motion during cardiac systole and diastole.[6]
The natural history of syringomyelia remains poorly understood; its unpredictable and variable nature complicates prognosis. Factors such as etiology, the extent of neurological deficits, the size and location of the syrinx cavity, a syrinx diameter exceeding 5 mm, and associated edema can predict rapid deterioration. The rarity of the condition, its variable progression, and short follow-up periods challenge the assessment of treatment outcomes.[7]
Myelopathy, a major complication of syringomyelia, can lead to severe conditions such as spasticity, which may progress to paraplegia or quadriplegia, along with complications like decubitus ulcers, recurrent pneumonia, and bowel and bladder dysfunction.[8]
CASE REPORTA 50-year-old female with a history of hypertension and low back pain, attributed to L4-L5 and L5-S1 lumbar spondylolisthesis, presented with progressively worsening central spinal cord syndrome over the past year. Initially diagnosed in 2018 with spondylolisthesis and pain in both legs, particularly the left leg, she was treated with analgesics and corticosteroids, but her symptoms persisted. The pain eventually spread to the thoracic region and chest.
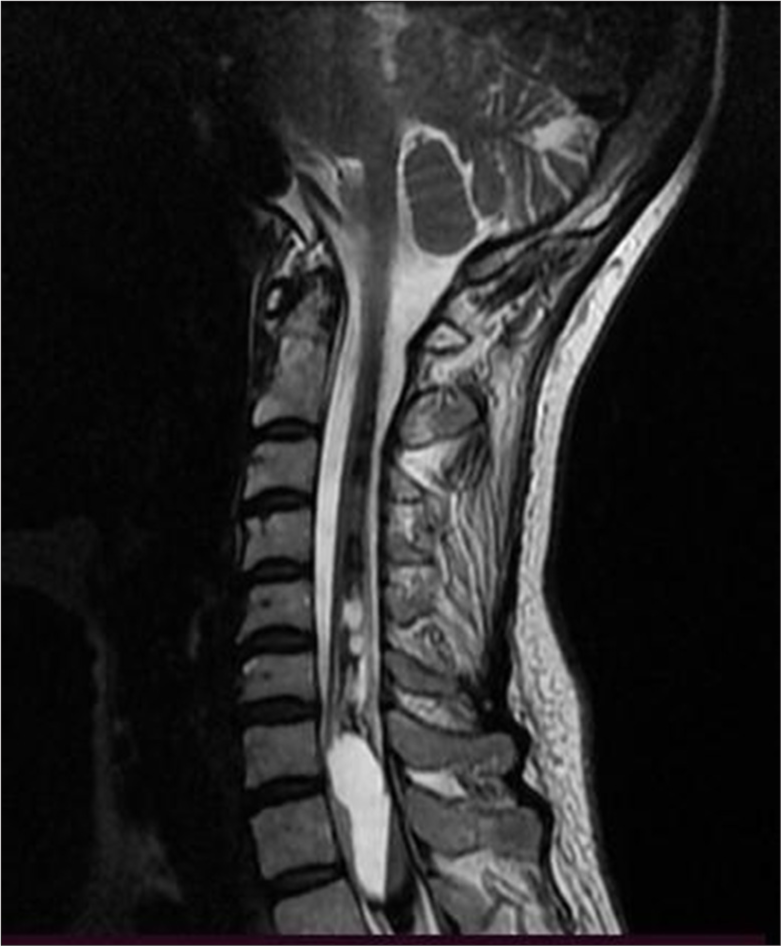
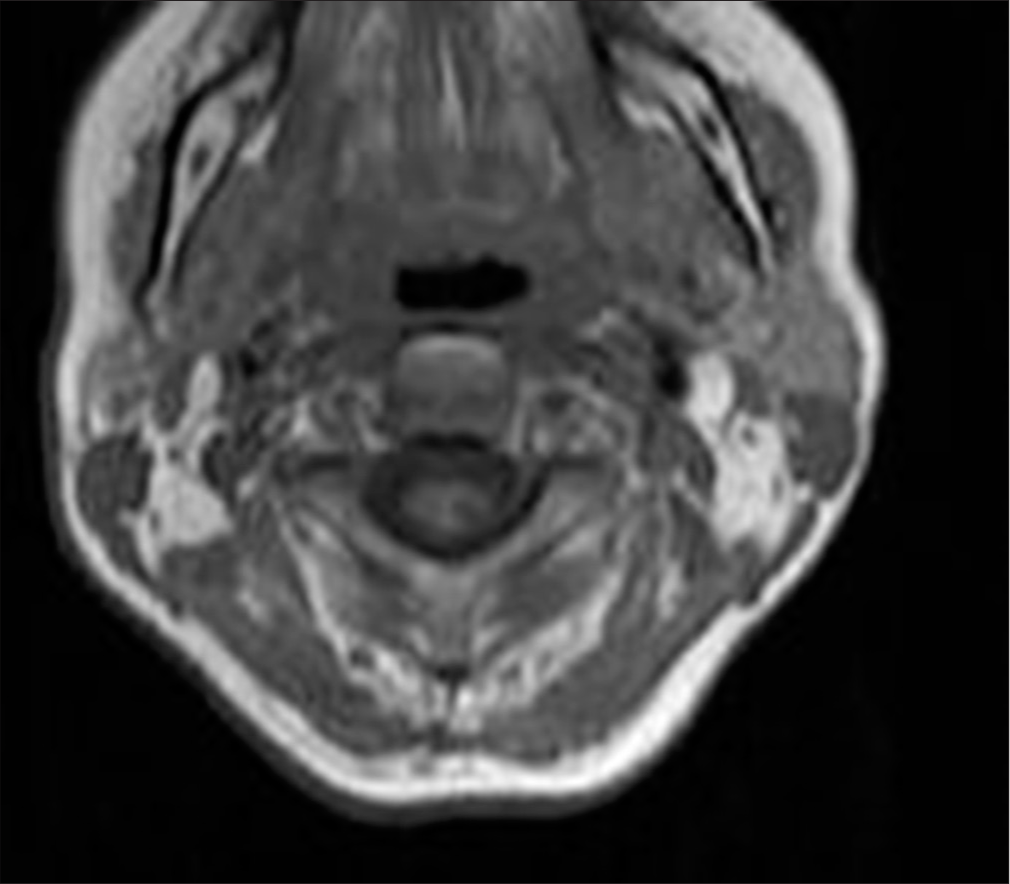
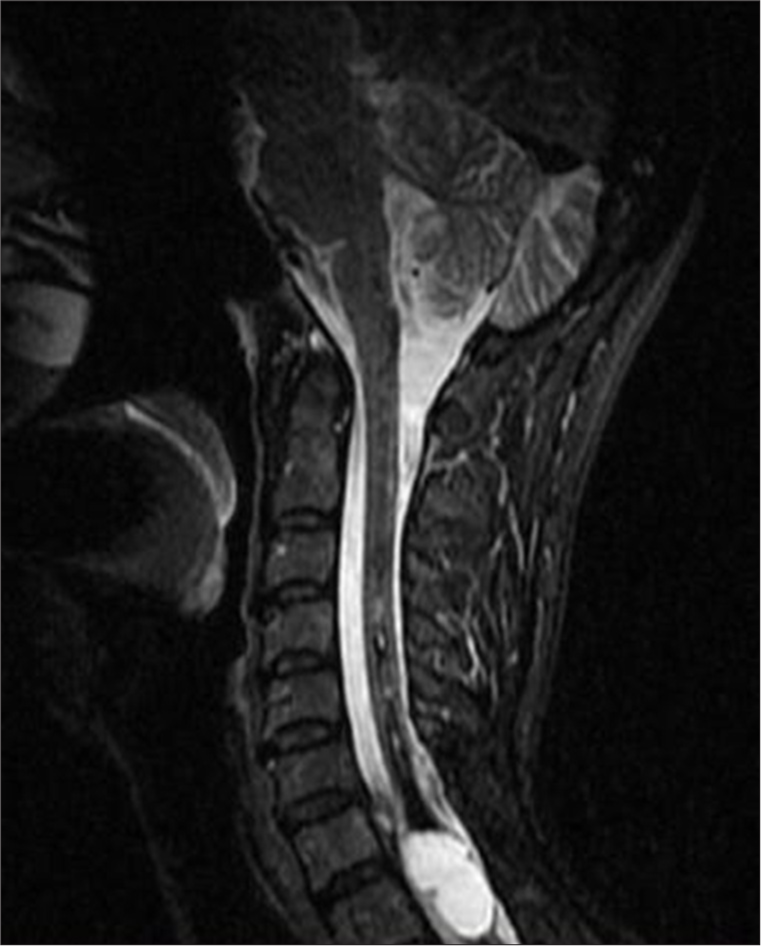
MRI of the cervical spine revealed an extensive T1-T2 intramedullary lesion with an intramedullary hematoma extending into the ependymal canal, accompanied by hemato/syringomyelia. The lesion measured 12 × 36 mm and was located between the C7 and Th3 vertebral levels, with a clearly visible fluid-fluid level [Figure 1]. Neurological examination revealed increased spasticity in the central spine, with lower extremity strength graded 2/5 on the right and 2.5/5 on the left, a sensory level at T2–3, and minimal involvement of the upper C8 roots. Marked osteotendinous reflexes were noted (3+ on both sides), along with clonus pedis and urinary retention.
Export to PPT
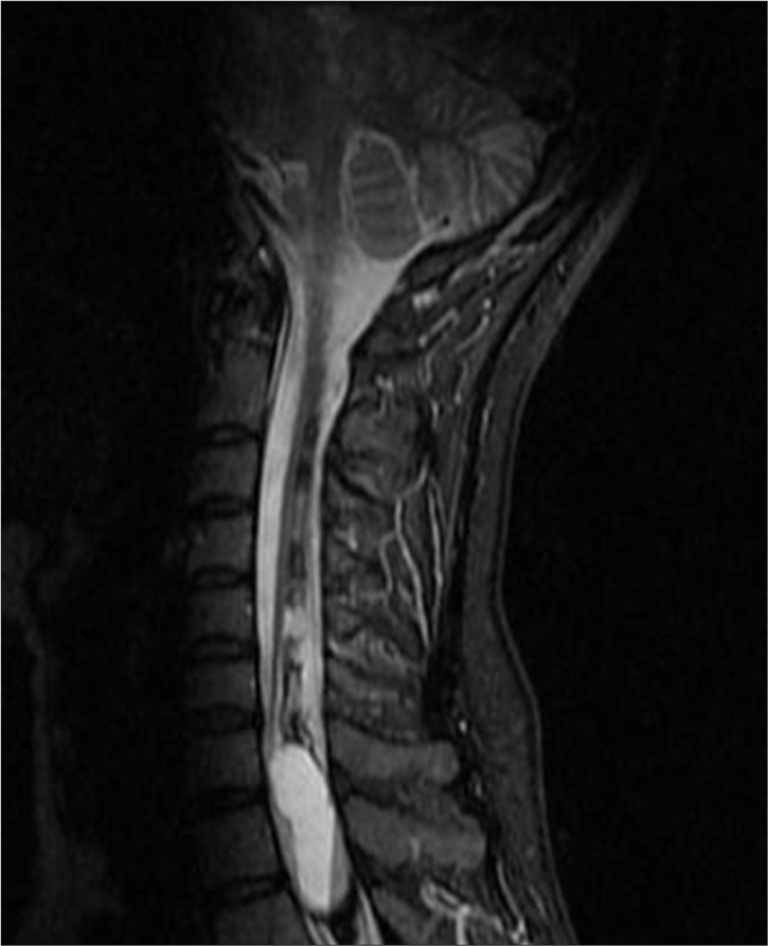
In 2018, an MRI showed an elongated lesion in the distal cervical medulla and upper dorsal spinal cord, exhibiting high-signal intensity on T2-weighted imaging [Figure 2] and heterogeneous hypointensity on T1-weighted images (T1WIs). These findings were consistent with hemato/syringomyelia. A more detailed short tau inversion recovery (STIR) sequence confirmed the fluid-fluid level between C7 and Th3 [Figure 3]. Despite inconclusive digital subtraction angiography, suspicion of a vascular anomaly remained.
Export to PPT
Export to PPT
Surgical intervention was recommended, and in 2019, the patient underwent a C7-T2 laminectomy and minimal midline T1 myelotomy. The intramedullary fluid was yellow-red, with clotted blood and hemosiderin deposits. A partially calcified lesion was identified in the proximal part of the cavity, and a gross total resection (GTR) was successfully achieved. Postsurgery, MRI imaging showed a reduction in the size of the syrinx and a noticeable improvement in the patient’s symptoms [Figure 4].
Export to PPT
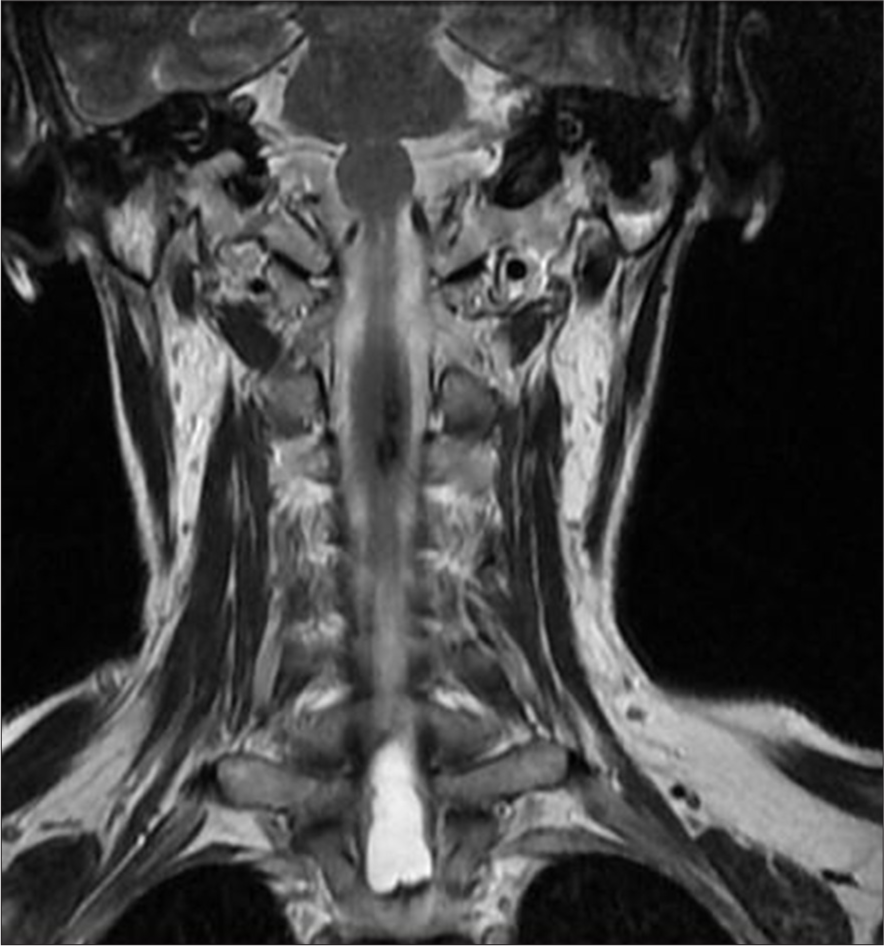
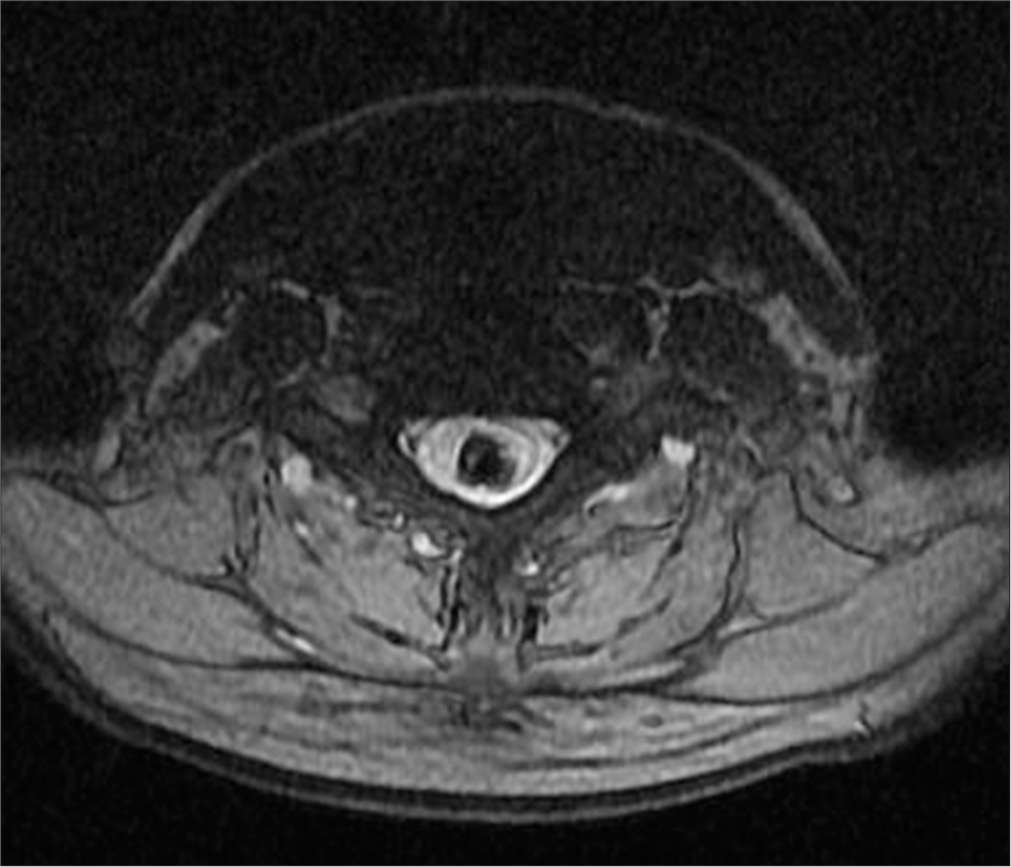
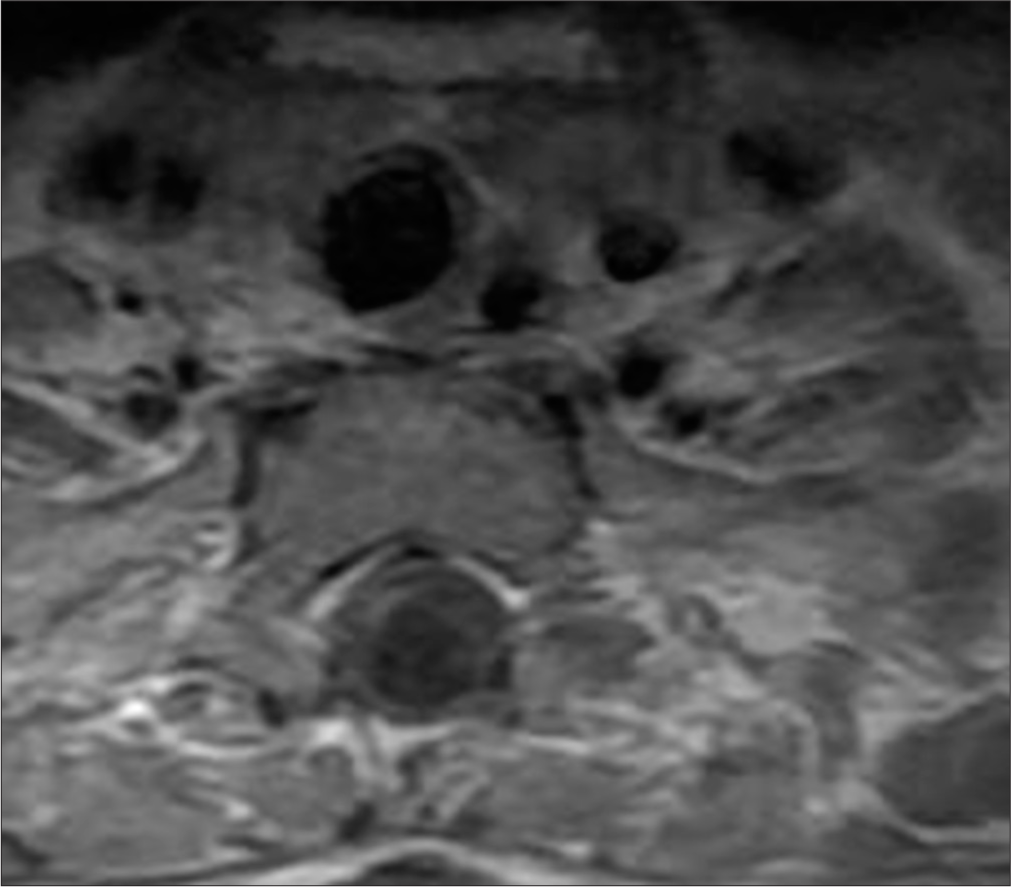
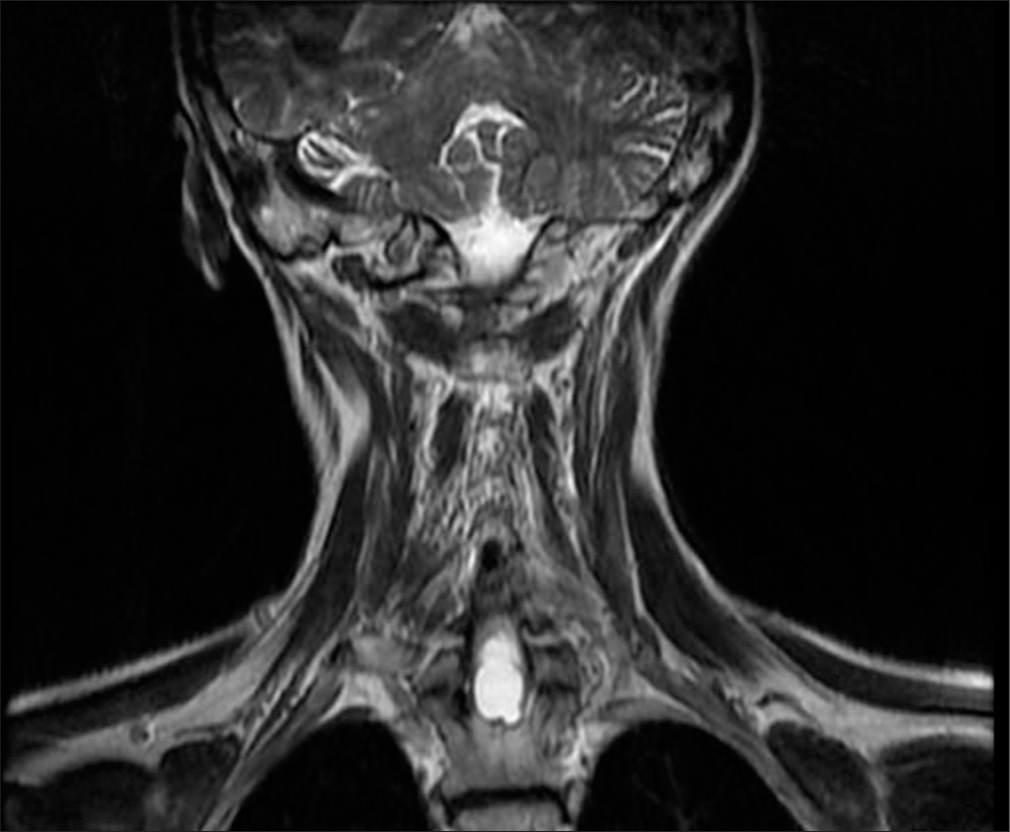
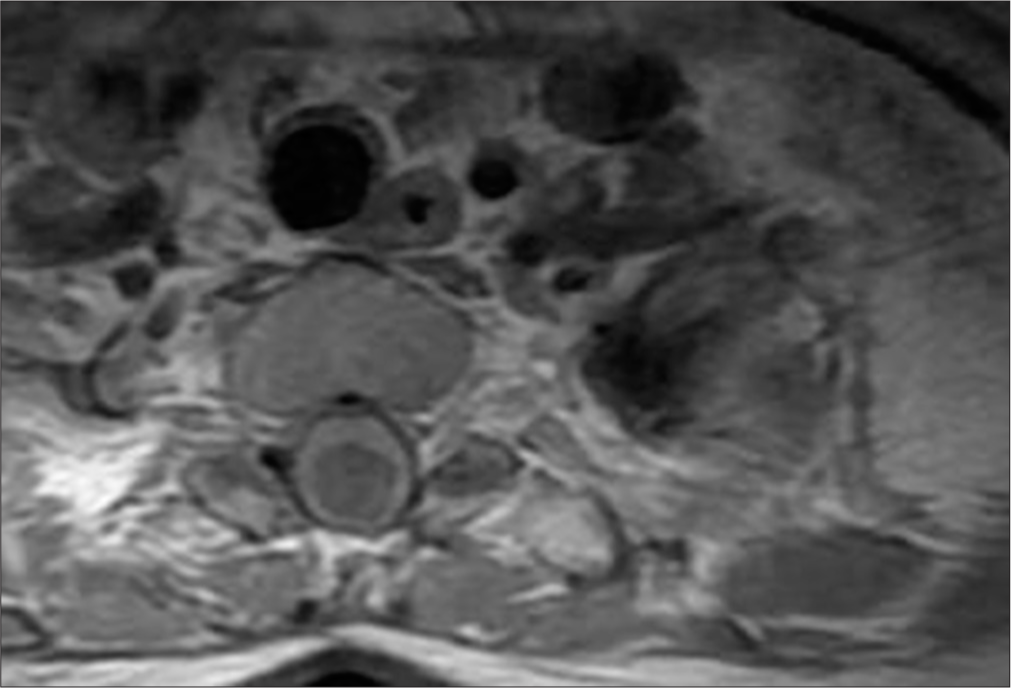
Post-operative MRI revealed minimal syringomyelia in the cervical spinal cord, with decreased T2 signal intensity and thickness of the cervical spinal cord [Figure 5]. A multilobulated expansile intramedullary lesion was still noted between the Th1-Th2 vertebrae, measuring 14 × 26 mm, with peripheral contrast enhancement indicating a vascular malformation [Figures 6 and 7]. Follow-up imaging showed further reduction in the spinal cord thickness and changes consistent with postoperative effects [Figures 8 and 9].
Export to PPT
Export to PPT
Export to PPT
Export to PPT
Export to PPT
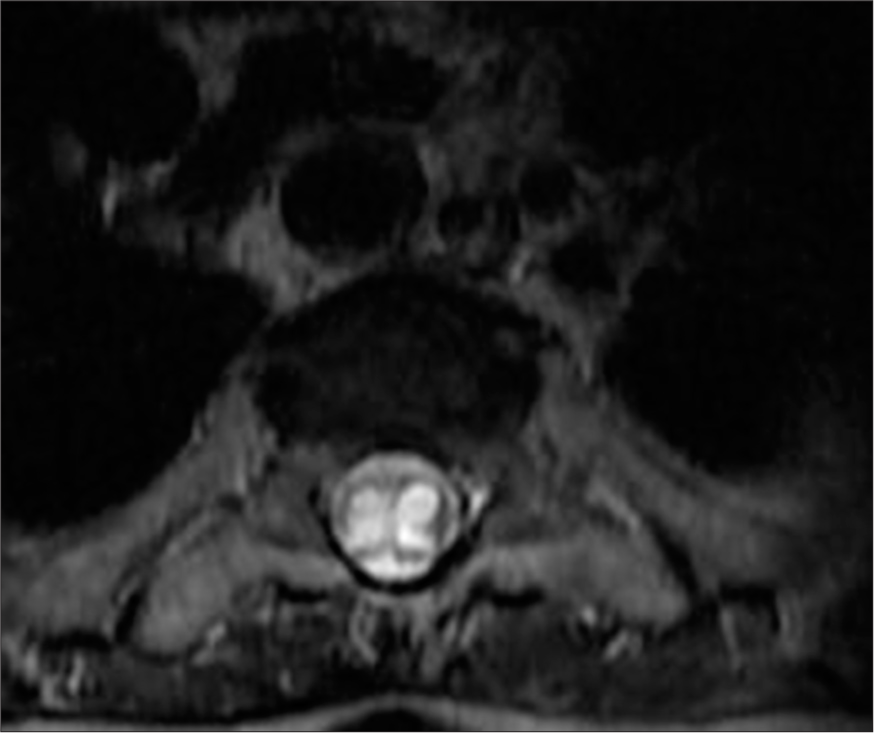
The patient’s neurological condition improved moderately after surgery, with partial recovery of motor function and reduction of sensory deficits. A follow-up MRI showed that the remaining expansile hyperintense lesion had stabilized; though some residual syringomyelia was still present [Figure 10].
Export to PPT
DISCUSSIONThis case involved a complex presentation of syringomyelia combined with hematomyelia, which led to notable neurological symptoms such as spastic paresis, clonus pedis, and urinary retention. MRI findings were consistent with giant hemorrhagic syringomyelia with extensive longitudinal involvement of the spinal cord and the large diameter of the cavity, displaying characteristic low-signal intensity on T1WI, high-signal intensity on T2-weighted images, and hemorrhagic signal characteristics.
Management of this intricate condition required a neurosurgical approach, including C7-T2 laminectomy and minimal midline T1 myelotomy. The intramedullary fluid was yellow-red, containing clotted blood and hemosiderin deposits. A partially calcified lesion was identified in the proximal part of the syrinx cavity, and a gross total resection (GTR) was successfully performed. Post-surgery, there was a noticeable improvement in the patient’s symptoms, underscoring the importance of prompt and appropriate intervention in managing syringomyelia and hematomyelia effectively.[9,10]
Intrasyringeal hemorrhage, where bleeding occurs within a pre-existing syrinx cavity, was first described by Gowers in 1904 and later termed “Gowers’ syringeal hemorrhage” by Wilson in 1955. Such hemorrhages lead to increased pressure within the syrinx cavity, resulting in potentially severe and complex clinical presentations.[11-13]
Although syringomyelia itself is relatively rare, its association with hemorrhage, as illustrated in this case, is even less common. The management of giant hemorrhagic syringomyelia presents distinct diagnostic and therapeutic challenges that necessitate a multidisciplinary approach. Differential diagnoses for syringomyelia include Chiari malformation, spinal cord tumors, and post-traumatic causes.[12]
Hematomyelia, or intramedullary spinal cord hemorrhage, is an uncommon cause of myelopathy and can manifest in various forms, including acute, subacute, stepwise, or chronic presentations. Typically, hematomyelia presents acutely with rapid neurological decline and can result in severe spinal cord syndrome.[14] Prognosis following spinal cord compression is closely related to the extent of neurological impairment and the duration between the onset of symptoms and surgical decompression.[9,10,15]
Early diagnosis and intervention are crucial, as hematomyelia can lead to permanent disability if not addressed promptly. MRI is preferred over conventional myelography or computed tomography (CT) for diagnosing such conditions.[16] Steady-state free precession MRI sequences, as fast imaging employing steady-state acquisition or CISS offer superior contrast resolution between CSF and neural tissue, making them especially valuable for delineating syrinx cavities, detecting subtle septations, and identifying associated arachnoid adhesions or spinal cord tethering. These sequences are highly sensitive for visualizing fluid-filled structures and their relationship with surrounding anatomy. Moreover, dynamic MRI, specifically cine phase-contrast cerebrospinal fluid (CSF) flow studies are particularly valuable for assessing the presence and degree of obstructive CSF flow dynamics associated with syringomyelia. By visualizing and quantifying CSF pulsations in real time, cine MRI can help identify flow disturbances at the foramen magnum or along the spinal canal, which may contribute to syrinx formation or progression. Such information is crucial for determining the need for surgical intervention and tailoring treatment strategies. Moreover, the fluid-fluid level seen on MRI in our case could represent a chronic hemorrhagic process or layering of cellular content, which may complicate further the diagnosis of syringomyelia.
In addition, CT myelography remains an important adjunctive modality, particularly in cases where MRI is inconclusive or contraindicated. It provides high-resolution imaging of the subarachnoid space and is especially useful in identifying CSF flow obstruction or adhesions that may contribute to syrinx formation. Incorporating these advanced imaging modalities into the diagnostic workflow allows for a more comprehensive evaluation and can guide therapeutic decision-making with greater precision. Our study has a limitation, due to not performing a spinal angiogram to clarify the possibility of an underlying vascular lesion such as an arteriovenous malformation, AVF, or cavernoma, which cannot be definitively excluded based on the imaging and clinical data available.
Further, clinical cases presenting with progressive neurological deficits and hemorrhagic cavity formation warrant urgent surgical intervention. Thus, neurosurgical intervention remains the primary treatment strategy to address the bleeding source and to restore neurological function. Timely surgical intervention significantly improves disease outcomes, reducing both mortality and disability.[15,17]
This case emphasizes the critical importance of early diagnosis, a multidisciplinary approach, and long-term follow-up in managing giant hemorrhagic syringomyelia and hematomyelia. Continued research is necessary to enhance understanding of these complex neurological conditions and to explore new treatment strategies.
In conclusion, this case underscores the significance of recognizing and managing giant hemorrhagic syringomyelia and hematomyelia, highlighting the benefits of early intervention and comprehensive care in optimizing patient outcomes.
CONCLUSIONSyringomyelia and giant hemorrhagic hematomyelia are intricate neurological conditions requiring meticulous management and long-term care. Advances in diagnostic imaging, particularly MRI, have proven crucial in the evaluation and treatment of these disorders. Early and precise diagnosis through MRI, coupled with timely surgical intervention, significantly enhances patient outcomes. The sophistication of modern imaging techniques enables detailed assessment of these conditions, facilitating early detection and optimal treatment planning. Thus, the judicious use of MRI and thorough interpretation are vital for achieving favorable results and improving patient prognosis in cases of syringomyelia and giant hemorrhagic hematomyelia.
Comments (0)