Understanding the potential of increased physical activity and exercise as vital components of a broadly understood healthy lifestyle, in disease prevention and treatment, is of increasing interest. Studies suggest that exercise-based interventions may have a beneficial effect on central nervous system (CNS) functionality. It was determined that exercising regularly might contribute to decreasing the risk of most CNS disorders, such as mood disorders and neurodegenerative diseases (Wang et al., 2024; Rai and Demontis, 2022). Improvement in mood and cognition (including memory and learning) in both patients and animal models of depression (Chen et al., 2017), Alzheimer’s disease (De la Rosa et al., 2020; Lourenco et al., 2019; Weinstein et al., 2014; Gaitán et al., 2021) or Parkinson’s disease (Zhou et al., 2017; Zhang et al., 2023) following exercise. The benefits of exercise have been determined to reshape the brain structure of depression patients, promote behavioral adaptation changes, and maintain the integrity of hippocampal regions (Zhao et al., 2020) or connectivity by enhancing neurogenesis and synaptic plasticity, and changing metabolism and vascular function (Cotman et al., 2007).
An improvement in behavior and cognitive abilities was determined to correspond with upregulated levels of neurotrophic factors and markers of synaptic plasticity, accompanied by downregulated proinflammatory mediators’ expression during exercise (Rai and Demontis, 2022; Mattson, 2012; Abdulghani et al., 2023). Many studies have examined the role of myokines in the context of beneficial effects of exercise on brain function, the aging process, and neurodegeneration. Myokines, muscle-secreted growth factors, and cytokines were suggested to play an important role in mediating the positive effects of exercise on the CNS (Rai and Demontis, 2022; Hutton et al., 2015; Farmer et al., 2004) via encompassing a variety of exercise-induced signaling molecules release, as a result of aerobic or strength training (Pedersen and Febbraio, 2008; Trillaud et al., 2023; Chow et al., 2022). Approximately a thousand proteins produced by skeletal muscles have been identified, but only parts have been well characterized (Pang et al., 2021; Florin et al., 2020). Therefore, skeletal muscles showed a significant endocrine capacity to impact many tissues and organs suggesting that also the CNS might be a target of muscle-initiated signaling. In addition to endocrine signaling through the circulation, direct muscle-to-nerve connections may also provide a route for signaling from skeletal muscle to the brain (Pedersen, 2019; Pedersen et al., 2007; Chen W. et al., 2021; Kim et al., 2019). Adipokines and hepatokines are also involved in the mediation of the beneficial effects caused by exercises, via promoting neurogenesis, synaptic plasticity, improving cognitive functions and energy metabolism, highlighting the existence of muscle-brain signaling (Trillaud et al., 2023; Lauretani et al., 2020; Vivar et al., 2013).
Acting by autocrine and paracrine signaling, myokines intercede in crosstalks between skeletal muscles and other organs, such as the brain (Hutton et al., 2015; Ding et al., 2006; Nakajima et al., 2010), adipose tissue (Boström et al., 2012; Tanimura et al., 2022), bones (Shahabi et al., 2021), liver (Nakajima et al., 2010; Tanimura et al., 2022; Chan et al., 2024), heart (Ho et al., 2018) or intestines (Lee et al., 2023; Liu et al., 2023). Their role is also to mediate metabolic processes, thereby contributing to the growth and regeneration of skeletal muscle cells (Rai and Demontis, 2022; Duzel et al., 2016; Jodeiri Farshbaf and Alviña, 2021), as well as enhancing muscle hypertrophy development (Erickson et al., 2013; Li et al., 2022; Delezie et al., 2019). Research shows that the level of myokines in muscles varies depending on their structure and specific function. In rodent studies, the greatest emphasis is put on the calf muscles (soleus, gastrocnemius) (Florin et al., 2020; Augusto et al., 2004), and the quadriceps muscles (Erickson et al., 2013; Li et al., 2022; Zakharova et al., 2021). It is also suggested that the type of incorporated training predisposes muscles to different myokine secretion profiles (Molanouri Shamsi et al., 2015). The endurance training was determined to cause an increase in IL-6 concentration in slow-twitch muscles (soleus) (Isanejad et al., 2015), while after strength training, IL-6 expression was comparable in both types of muscles: slow-twitch soleus and fast-twitch flexor hallucis longus (Molanouri Shamsi et al., 2015).
The exercise was determined to induce a favorable myokine profile and stimulate skeletal muscle cells to produce regulatory factors such as cytokines, hormones, growth factors, and exosomes (Chow et al., 2022; Chen W. et al., 2021; Blume and Royes, 2024; Pedersen, 2009; Vints et al., 2022). This beneficial myokine profile has been associated with effects on lipolysis and β-oxidation, glucose uptake and metabolism, or angiogenesis (Ji et al., 2024; So et al., 2014; Giudice and Taylor, 2017). Although some circulating factors cannot pass the blood-brain barrier (BBB), they still might influence the brain by binding to receptors located on the endothelial cells of the BBB (Zhang et al., 2018). As a result of increased physical activity, the following main myokines are secreted: interleukins (IL-4, -6, -7, -15) (Zakharova et al., 2021; Roca-Rivada et al., 2012), insulin-like growth factor-1 (IGF-1) (Erickson et al., 2013; Munive et al., 2016; Munive et al., 2019), brain-derived neurotrophic factor (BDNF) (Hutton et al., 2015; Ding et al., 2006; Farmer et al., 2004; Chan et al., 2024), fibroblast growth factor 21 (FGF21) (Mashili et al., 2011). Importantly, it has been established that irisin/FNDC5 (fibronectin-domain III containing 5), IGF-1, IL-6, cathepsin B (CTSB), and BDNF might cross the BBB, subsequently contributing to neuronal functioning improvement by modulating synaptic plasticity (Pedersen, 2019; Jodeiri Farshbaf and Alviña, 2021; Hashimoto et al., 2021; Ni et al., 2022; Jiang et al., 2020).
The following article reviews the information available regarding the main myokines determined to cross BBB, specifically addressing the association between exercise-induced myokine release and mood disorder development. This review summarizes only the results of experimental research that might support the role of those myokines in muscle-brain crosstalk (Figure 1).
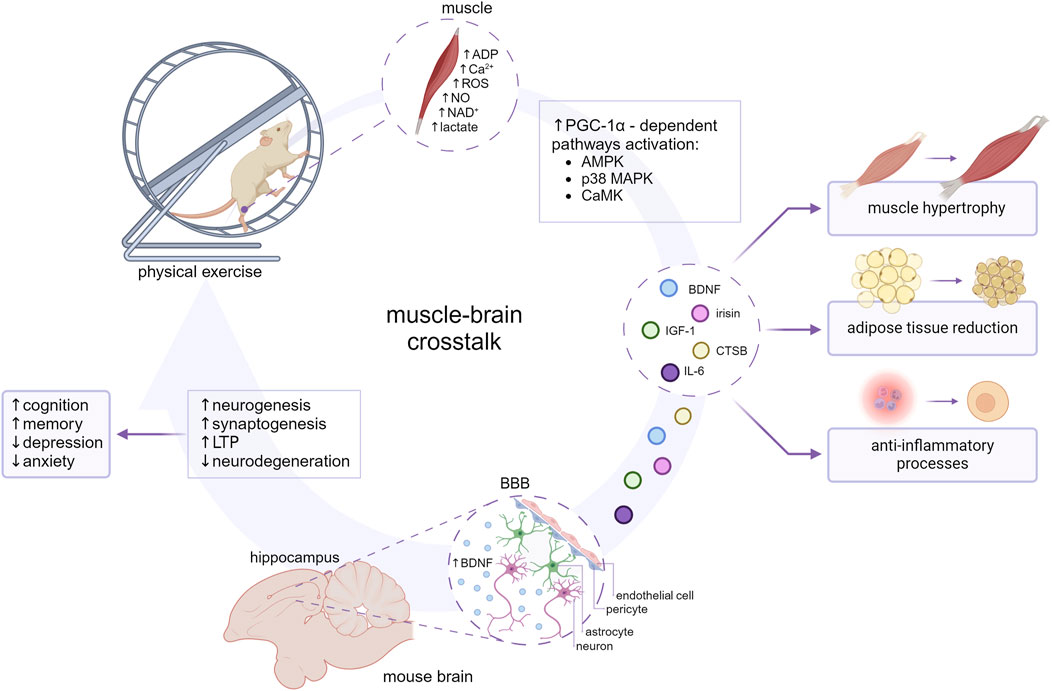
Figure 1. The impact of myokines on the muscle-brain crosstalk. The molecular mechanisms involved in the release of myokines seem to coincide with molecular adaptation to exercise and are largely mediated by transcription factors such as peroxisome proliferator-activated receptor (PPAR)-gamma coactivator-1α (PGC-1α). PGC-1α-dependent myokines (BDNF, irisin, CTSB, IL-6, IGF-1) are subsequently released into the blood circuitry and influence an array of metabolic processes. At the cellular level myokines might ameliorate inflammation, impact lipid and carbohydrate metabolism, and enhance angiogenesis and mitochondrial biogenesis. BDNF, CTSB, IL-6, IGF-1, and irisin are suggested to cross the BBB and reach the brain parenchyma, contributing to neurogenesis, synaptogenesis and long-term potentiation (LTP) enhancement, and consecutive improvement in cognition, memory, and behavioral alterations. Created with BioRender.com.
2 Myokines as a messenger of skeletal muscleMuscle contractions induce many physiological and metabolic adaptations in other organs, which are not mediated by the nervous system. Therefore, these processes might be mediated by myokines - factors expressed, produced, and secreted during exercise by muscle fibers (Pedersen, 2019; Pedersen et al., 2007; Chen W. et al., 2021; Kim et al., 2019). Although the myokines are generally characterized as cytokines released by myocytes, it should be emphasized that other muscle cell types were established with continuous cytokines’ expression as well (reviewed by Peake et al., 2015). In vitro studies on C2C12 myoblasts (Chan et al., 2024), L6 myoblasts (Moon et al., 2016), or myotubes provide insights into which myokines might be specific for which cell types. Based on the cell culture studies, myoblasts-mesodermal precursors of myocytes-were characterized with persistent expression of IL-8, IL-12, IL-15 or TGF- β. Moreover, the expression of myoblast-located myokines was found lower than in myotubes. Interestingly, some myokines were demonstrated to have fiber-specific expression. IL-6 expression was found the highest in type II muscle fibers as a result of endurance exercise. On the other hand, it was suggested that VEGF expression might be found mostly in the subsarcolemmal sarcoplasm of type I, IIa, and IIb muscle fibers after endurance exercise (reviewed by Peake et al. (2015)).
Experimental studies indicate that the communication between the brain and muscles is reshaped by exercise via the activity of many signaling pathways and the consequent modulation of the expression and secretion of several myokines with neuroprotective functions. Mutual interactions between myokines, especially BDNF and IGF-1, and the brain occur through pathways called the “muscle-brain axis” (Duzel et al., 2016; Lee et al., 2024; Burtscher et al., 2021) or the “muscle-brain crosstalk” (Pedersen, 2019; Chen W. et al., 2021; Lauretani et al., 2020; Blume and Royes, 2024; Ibeas et al., 2021). This connection is often observed when muscular damage (Li et al., 2019), and thus low levels of myokines, are associated with impaired cognitive functioning following sarcopenia (Blume and Royes, 2024; Jo et al., 2022; Choi et al., 2024; Markowska et al., 1998). Katz et al. (2022) established similarities in AKT (protein kinase B) and mTOR (mammalian target of rapamycin) pathways activity in the hypothalamus and muscles as a result of both, voluntary and forced exercise. An increase in AKT and a decrease in mTOR activity in muscles were also observed in the hypothalamus, supporting the idea of muscle-brain coordination existence. It was also confirmed by a study unveiling the impact of musclin on improved hypothalamic signaling and concomitant amelioration of depressive-like behaviors (Ataka et al., 2023). Moreover, irisin was found to act as a regulator of monoamine levels in subcortical brain regions, associated with vulnerability to developing neuropsychiatric disorders (Yardimci et al., 2023). Other evidence indicated a possible role of exercise-induced increased serum- and glucocorticoid-inducible kinase 1 (SGK1) expression and its neuroprotective features on improvement in cognition (Liu et al., 2024).
3 Exercise-induced myokine release regulationThe molecular mechanisms involved in the release of myokines seem to coincide with molecular adaptation to exercise and are largely mediated by transcription factors such as PGC-1α and PPAR-β, and metabolic sensors such as 5′-AMP activated kinase (AMPK). Recent studies showed that the activity of myokines is mostly regulated by signaling pathways related to the PGC-1α protein (Chan et al., 2024; Fan et al., 2017; Calvo et al., 2008). Its expression is induced by calcium ions released as a result of exercise, as well as by energy obtained from adipose tissue in cold environmental conditions/temperatures (Puigserver et al., 1998; Lin et al., 2002). The increase in PGC-1α concentration was observed as a result of strength training in both young (10 weeks) and middle-aged (50 weeks) rats (Jung et al., 2015). PGC-1α was suggested to induce mitochondrial biogenesis, mainly during aerobic training in slow-twitch muscles, while the PGC-1α4 release was established during strength training leading to muscle hypertrophy (Lin et al., 2002; Miller et al., 2019; Villena, 2015). An increase in the muscle PGC-1α alone, with no additional exercise interventions, did not induce such effects, as confirmed by studies on transgenic mice with overexpressed PGC-1α (Karlsson et al., 2019). Moreover, this overexpression was demonstrated to lead to metabolic disorders such as insulin resistance (Rai and Demontis, 2022; Karlsson et al., 2021). Mice overexpressing PGC-1α were characterized by increased profiles of cytochrome C, cytochrome oxidase II, and cytochrome IV oxidase (Mirebeau-Prunier et al., 2010; Wende et al., 2007), and showed improved performance functions such as increased peak oxygen uptake VO2 and covering longer distances at higher speeds while running on a treadmill (Calvo et al., 2008). Skeletal-muscle-specific knockout animals for PGC-1α were shown to suffer from a reduced endurance capacity as well as other signs of pathological inactivity (Handschin et al., 2007a; Handschin et al., 2007b). Increased expression of genes encoding oxidative phosphorylation enzymes was demonstrated in the gastrocnemius muscle, which indicated an improvement in lipid metabolism (Calvo et al., 2008; Wende et al., 2007). On the other hand, insulin sensitivity and glucose uptake were not changed in transgenic mice compared to control mice (Calvo et al., 2008).
In addition to PGC1α-mediated responses, a key component of exercise is the depletion of energy stores and the consequent activation of the AMPK, which senses AMP/ADP (adenosine monophosphate/adenosine diphosphate) concentrations. Mitochondria biogenesis, a highly coordinated process that requires multiple cellular events, including transcription of two genomes (nuclear and mitochondrial) (Hood et al., 1989; Cisterna et al., 2023), is strongly induced by the disturbance of metabolic homeostasis generated by exercise training. This dysregulation activates the main signaling pathways such as AMPK, MAPK (mitogen-activated protein kinase), PKA (protein kinase A), and also increased cytoplasmic free Ca2+, thereby impacting the expression of myokines (Hawley et al., 2014; Angulo et al., 2020; Reisman et al., 2024; Brooks, 2018). Changes in the mitochondrial oxidative capacity accompanied by an increase in the number of mitochondria and mitochondrial enzyme activity in response to exercise seem to be the main determinant (Laurens et al., 2020).
Here, we introduce some of the myokines that have been suggested to cross BBB and have frequently been linked to cognition, including BDNF, irisin, CTSB, IGF-1, and IL-6 (Table 1).
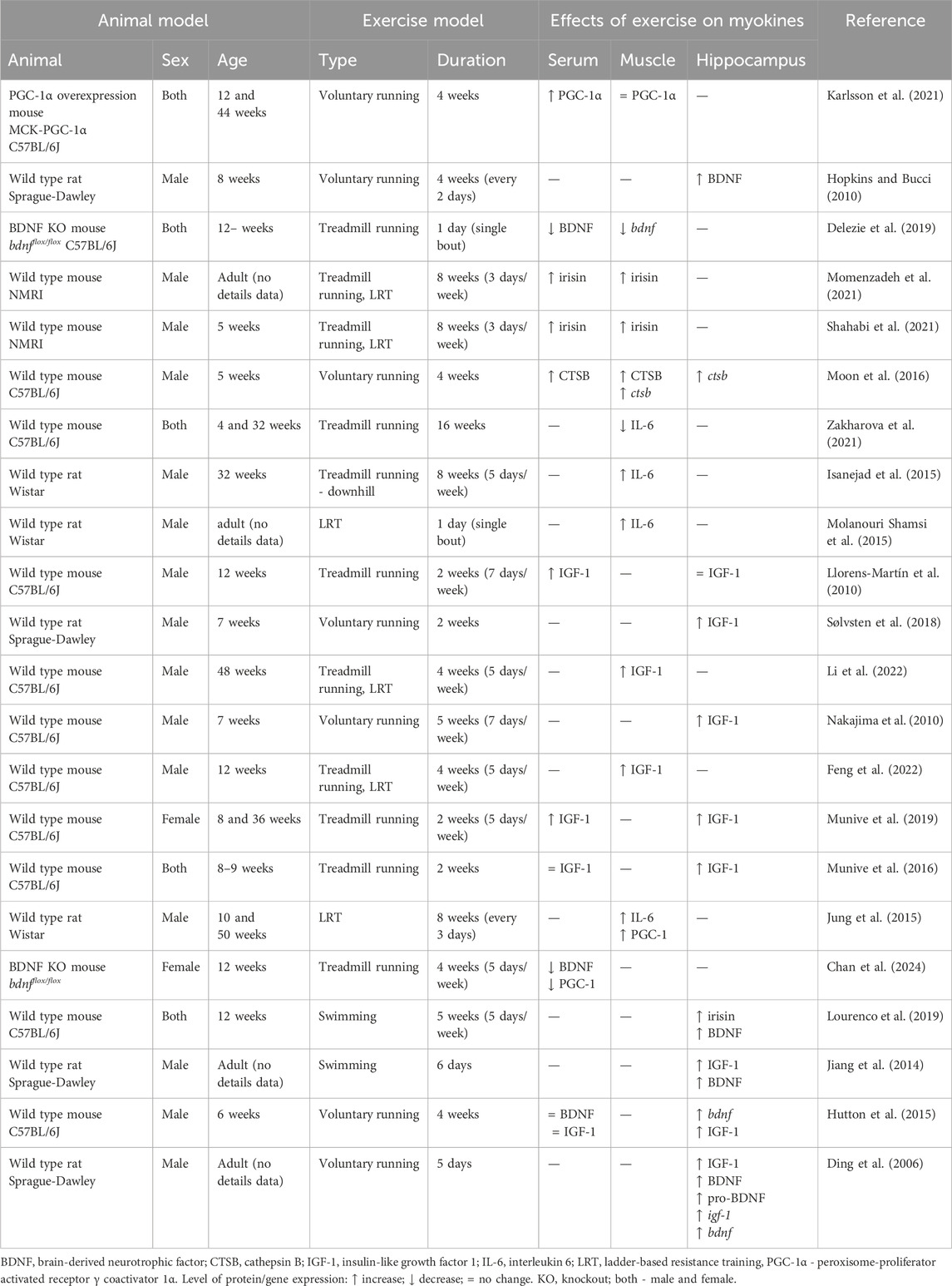
Table 1. List of animal studies that investigate exercise myokines expressed and/or secreted by skeletal muscle which act on muscle-brain tissue crosstalk.
4 Myokines linked with muscle-brain crosstalk4.1 Brain-derived neurotrophic factor (BDNF)BDNF is one of the most thoroughly studied neurotrophins and the most studied myokine. Its well-described functions as neurotrophin, include promoting the formation and maturation of neurons, enhancing neurogenesis and neuroregeneration, and reducing apoptosis through tropomyosin-related kinase receptor B (TrkB) (Gaitán et al., 2021; Rezaee et al., 2023; Yang et al., 2019). Its beneficial effect on cognitive functions is manifested by intensifying synaptic plasticity, leading to LTP (Vints et al., 2022; Zhou et al., 2023; Binder and Scharfman, 2004). The exercise-induced upregulation of hippocampal BDNF expression was confirmed in many animal studies (Duzel et al., 2016; Vints et al., 2022; Zhou et al., 2023; Sorrells et al., 2018; Leal et al., 2014; Lu et al., 2014; Jiang et al., 2014). The increased concentration of BDNF was noted in mice serum and hippocampus after exercise, while a greater increase was observed during breaks in exercise periods (Hutton et al., 2015; Ding et al., 2006; Farmer et al., 2004; Jo et al., 2022; Lei et al., 2024). Increased levels of BDNF in the hippocampus were also demonstrated in the offspring of mice running on a treadmill (Kim et al., 2024). Importantly, an exercise-induced increase in hippocampal BDNF expression was associated with functional changes, for instance, a reduction of anxiety- and depressive-like behaviors and improved cognition in voluntary-running rats (Hopkins and Bucci, 2010; Eldomiaty et al., 2017; Yau et al., 2012; Bechara and Kelly, 2013; Lee et al., 2012).
The neuronal mechanisms involved in exercise-induced release of neurotransmitters leading to the upregulation of bdnf expression in the brain are well known. During exercise, membrane depolarization and neurotransmitter binding lead to ligand-gated and voltage-gated Ca2+ channels (L-VGCC) activation at the cell membrane. Increased Ca2+ concentration activates signaling systems including the MAPK, the Ca2+/calmodulin-activated kinase (CAMK), and Ca2+−sensitive adenylate cyclase/PKA, leading to CREB (cAMP response element-binding protein) phosphorylation, and BDNF transcription. In addition to membrane depolarization, glutamatergic transmission mediated by AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor) and NMDA receptors (N-methyl-D-aspartate receptor), plays an important role in this process (Nishijima et al., 2012).
Recently, it was proposed that cerebral mechanisms involved in exercise contribution to BDNF overexpression and concomitant positive effect on neurogenesis might be associated with exercise-induced metabolic factors (e.g., ketone bodies, lactate) and muscle-derived myokines (CTSB, irisin/FNDC5) (Moon et al., 2016).
CTSB and FNDC5 knockout mice showed decreased BDNF expression in the hippocampus and deteriorated cognitive functions, as demonstrated in a few behavioral tests: Morris Water Maze (MWM) (Moon et al., 2016), the Novel Object Recognition (NOR) (Lourenco et al., 2019), and the Open Field (Lourenco et al., 2019). Similarly, intraperitoneal administration of lactate with its inhibitor (MCT1/2) normalized the increase of BDNF in the hippocampus in mice subjected to exercise. Lower BDNF levels were associated with worsened memory in mice in the MWM test (Hayek et al., 2019) and NOR test (Hopkins and Bucci, 2010) compared to the control group.
Although BDNF is expressed primarily in the CNS, it might be also found in blood platelets (Radka et al., 1996; Karege et al., 2005), vascular endothelial cells (Bai et al., 2014), and organs associated with high metabolic activity, i.e., heart (Wang et al., 2018), kidneys (Tao et al., 2018), liver (Zhang et al., 2021), spleen (Prochnik et al., 2022), and skeletal muscles (Chan et al., 2024). As a result of exercise, the upregulation of BDNF in skeletal muscles was observed (Cuppini et al., 2007). Indeed, BDNF is differentially expressed in skeletal muscles depending on either homeostatic or pathological conditions (reviewed in Chevrel et al. (2006)). BDNF was linked with skeletal muscle regeneration. Its increased expression, followed by the activation and proliferation of satellite cells was observed after a muscle injury (Clow and Jasmin, 2010; Omura et al., 2005). Although many studies were associated with the role of BDNF in muscle development and function, the impact of muscle contraction on circulating BDNF levels is still understudied. One study suggests that based on an intravenous administration, BDNF might cross the BBB (Pan et al., 1998). Taken together, BDNF could be classified as a myokine, however, it is a matter of debate whether skeletal muscle could be a primary source of circulating BDNF levels. One of the proposed recent mechanisms involved the transport of BDNF in exosomes that could allow sustained release of BDNF in the brain (Yuan et al., 2017).
4.2 Irisin/FNDC5Irisin/FNDC5, a glycosylated type 1 membrane protein, has been identified as an important exercise-regulated factor that induces major metabolic benefits (Boström et al., 2012). Circulating irisin affects systemic homeostasis by regulating osteogenesis (Estell et al., 2020), reducing adipose tissue (Momenzadeh et al., 2021), leading to muscle hypertrophy (Reza et al., 2017), and inducing neurogenesis (Wrann et al., 2013). During exercise, mainly strength training, irisin induces osteogenesis, by increasing the expression of osteopontin and inhibiting the production of sclerostin (Kim et al., 2018). Increased bone density (Shahabi et al., 2021) and higher concentrations of irisin in the blood circuitry were found in mice subjected to resistance training in comparison to aerobic training or external administration of this myokine (Shahabi et al., 2021; Momenzadeh et al., 2021). Interestingly, external administration of irisin did not affect the bone density (Shahabi et al., 2021). Primarily discovered as a secreted factor from skeletal muscle, irisin expression was shown to be dependent on the PGC-1α activity (Wrann et al., 2013). Wrann et al. found increased irisin secretion in the quadriceps and the hippocampus of voluntary running mice, suggesting that irisin might pass through the BBB (Wrann et al., 2013). In the hippocampus, irisin was determined to affect the Akt, Erk, cAMP/PKA/CREB pathways, inducing an increase in BDNF expression with concomitant PGC-1α upregulation, thus influencing neuronal proliferation (Rai and Demontis, 2022; Momenzadeh et al., 2021; Wrann et al., 2013). Following the exercise, Val66Met polymorphism was associated with reduced expression of brain FNDC5 and BDNF in mice (Ieraci et al., 2016). Extending those findings is a study by Lourenco et al. showing the reduced brain levels of irisin in AD (Alzheimer’s disease) mouse models and further, stating that irisin might be a novel mediator of the beneficial effects of exercise on synapse function and memory in AD models (Lourenco et al., 2019). In addition, recent data shows that elevation of the circulating irisin by its peripheral delivery improved cognitive function in transgenic AD mouse models (APP/PS1), and even in wild-type mice (Islam et al., 2021). PFF α-syn mice (non-transgenic model of Parkinson’s disease (PD)) injected with irisin showed improved motor functions, reduced tyrosine hydroxylase activity, loss of dopamine receptors and dopaminergic neurons, and reduced amount of residing α-synuclein (Kam et al., 2022).
4.3 Cathepsin B (CTSB)Lysosomal cysteine protease CTSB is ubiquitously expressed throughout the body (Turk et al., 2012). Depending on the pH of the environment, it assumes the function of exopeptidase (acidic pH) or endopeptidase (neutral pH) (Schmitz et al., 2019; Yoon et al., 2022). CTSB normally functions in lysosomes thereby degrading proteins and maintaining cellular homeostasis. However, in pathological conditions, it is translocated to the cytosol, where it activates inflammatory processes, and induces cell death (Wang et al., 2023; Lambeth et al., 2021). CTSB is highly expressed in tumors thus it is used as a biomarker for imaging (Chen X. et al., 2021). CTSB located in lysosomes was determined to promote autophagy and immune response by trafficking TNF-α (tumor necrosis factor α)-containing vesicles (Ni et al., 2022; Ha et al., 2008). Autophagy is currently being studied in psychiatric conditions. It is well established that cathepsins are engaged in disease progression (Moon et al., 2016; Matthews et al., 2023). Kindy et al. demonstrated that CTSB knockout mice in the model of AD exhibit memory impairments (Kindy et al., 2012) and that the inhibition of CTSB promotes the degradation of β-amyloid in AD (Choi et al., 2013).
CTSB, recently discovered as myokine, was reported to increase in plasma in response to aerobic exercising in animal models (including voluntary wheel running mice and treadmill training monkeys) (Moon et al., 2016). Passing through the BBB, CTSB promotes BDNF expression in the hippocampus, contributing to neurogenesis and improving memory (Rai and Demontis, 2022; Jiang et al., 2020; Ibeas et al., 2021). It also initiates the growth of neurites and axons, thereby contributing to the nervous system development (Jiang et al., 2020). In a study by Moon et al. (2016) involving voluntarily running male mice, CTSB expression in the hippocampus was upregulated compared to the sedentary group. The authors suggested CTSB as a mediator of the effects of exercise on cognition. Consistently, CTSB concentration in the plasma and gastrocnemius muscles was also increased. Furthermore, intravenous administration of CTSB to CTSB knockout mice enhanced BDNF and doublecortin (DCX) expression in adult hippocampal progenitor cells (Moon et al., 2016).
Interestingly, CTSB activity was found to be tissue-dependent. Gornicka et al. suggested that overexpression of CTSB in adipose tissue might lead to increased lysosomal membrane permeabilization, promote inflammation, and upregulate autophagy. This initial event leads to reactive oxygen species (ROS) overproduction, mitochondrial dysfunction, and at the end to adipocyte death and macrophage infiltration (Gornicka et al., 2012). Dysregulation at the cellular level is associated with chronic inflammation in adipose tissue leading to glucose intolerance, hepatic steatosis, insulin resistance, cardiovascular disease, and type 2 diabetes (Araujo et al., 2018). Research by Daneshyar et al. (2023) determined increased CTSB expression in adipose tissue, in mice fed with HFD. However, these findings were not observed after subjecting mice to exercise. These inconsistent results might demonstrate the tissue-specific CTSB activity supported by studies covering autophagy-upregulated CTSB expression is concomitant with increased autophagy in adipose tissue. As a result of exercise, HFD-induced CTSB increase was lower than in the non-exercised group. The following outcomes might be associated with Bcl-2 (B-cell lymphoma 2 protein) being a substrate for CTSB synthesis, the expression of which is downregulated by exercise (reviewed in Araujo et al., 2018). Taken together, these outcomes may indicate the role of exercise in CTSB-mediated obesity prevention.
4.4 Interleukin-6 (IL-6)IL-6 is a pleiotropic cytokine with a broad spectrum of biological activity (Rose-John, 2012), produced by various cells, including immune cells, renal mesangial cells, adipocytes, muscle cells, and cancer cells (Kontny and Maśliński et al., 2009). It is involved in the immune response and inflammation during several disease progression (Sharif et al., 2018). In classic signaling, IL-6 binds to complex receptor IL-6Rα with glycoprotein gp130 expression on the cell membrane surface, majoring in immune cells (macrophages, lymphocytes) or skeletal myocytes (Sharif et al., 2018; Rose-John et al., 2017; Lin et al., 2023). By engaging soluble IL-6R (sIL-6R) and membrane-bound gp130, IL-6 induces a pro-inflammatory effect by trans-signaling (Rose-John et al., 2017; Petersen and Pedersen, 2006).
Regarding IL-6 impact on the CNS, it was determined to contribute to promoting neuronal survival (März et al., 1998; Pervaiz and Hoffman-Goetz, 2012). IL-6 and its receptors were found in brain endothelial cells (Eskilsson et al., 2014) and in brain structures like the hippocampus, cortex, thalamus, and clastrum (Vallières et al., 1997; Gadient and Otten, 1994; Schöbitz et al., 1993; Baier et al., 2009). It was suggested to cross the BBB (Banks et al., 1994) and to promote neurogenesis (influencing both neurons and glial cells) (Vallières et al., 2002). On the other hand, IL-6 might act completely opposing actions triggering either neuronal survival after injury or causing neuronal degeneration and cell death in disorders, such as AD (Kummer et al., 2021).
As a result of prolonged exercise, increased production and release of IL-6 in skeletal muscles classified this cytokine as myokine. Additionally, muscles are its important targets (reviewed in Muñoz-Cánoves et al., 2013). Souza et al. (2017) demonstrated the protective effects of exercising on the BBB permeability in an experimental model of autoimmune neuroinflammation. Experimental autoimmune encephalomyelitis (EAE) mouse model subjected to exercise showed decreased BBB permeability and downregulated cytokines expression, such as IFN-γ, IL-6, IL-17, and IL-1b (Souza et al., 2017). IL-6 levels were increased in treadmill-running mice while TNF-α levels were decreased in the hippocampus (Pervaiz and Hoffman-Goetz, 2012).
Muscle-induced IL-6 is responsible for metabolic effects and tissue regeneration (Kelly et al., 2009; Ahsan et al., 2022; Pedersen et al., 2007; Rose-John et al., 2017). Kelly et al. suggested that IL-6 released by gastrocnemius muscle in swimming rats might initiate the AMPK pathway and promote lipolysis, and glycogenolysis (Kelly et al., 2004). Jung et al. determined exercising induced an increase in IL-6 expression in the tibialis muscle in rats. The increase in slow-twitch muscle-derived IL-6 was accompanied by a decrease in TNF-α after endurance training in rodents (Isanejad et al., 2015; Jung et al., 2015). Similar observations were made in a study by Zuo et al., covering inflammation induced in muscles, after subjecting rats to running on a treadmill (Zuo et al., 2019). Importantly, it was proposed that exercise-induced IL-6 may be responsible for the long-term anti-inflammatory effects via enhancing epithelial cell division and growth and limiting apoptosis (Rose-John, 2012; Phillips and Fahimi, 2018; Kistner et al., 2022; Petersen and Pedersen, 2006).
It should be emphasized that IL-6 is highly synthesized and released post-exercise while insulin release is enhanced (Pedersen and Febbraio, 2008). However, IL-6 is also associated with obesity and insulin resistance (Pedersen and Febbraio, 2008). An increased higher IL-6 mRNA expression in the skeletal muscle was found in high-fat diet rats than in the control rats, and importantly it could be reduced to a much lower level with aerobic exercise (Gopalan et al., 2021). IL-6 was also shown to increase glucose uptake through mediating translocation of GLUT4 (glucose transporter 4) receptors in many tissues including muscle (Carey et al., 2006; Gopalan et al., 2021) and brain (Hussein et al., 2021; Vannucci et al., 1998). Moreover, Pearson-Leary et al. demonstrated that increasing hippocampal GLUT4 expression contributed to improving memory in exercising rats (Pearson-Leary and McNay, 2016). In addition, IL-6 was found to reduce TNF-α levels and enhance insulin release acting via the AMPK pathway (Oh et al., 2016). Thus, the level of IL-6 is closely related to the glucose metabolism that occurs in muscles during exercise (Isanejad et al., 2015; Helge et al., 2003; Pedersen, 2009).
4.5 Insulin-like growth factor –1 (IGF-1)IGF-1, a polypeptide similar structurally and functionally to insulin, acts as a mediator of most tissue effects of growth hormone (Vajdos et al., 2001; Laron, 2001). It is associated with neuronal development, neurogenesis, and synaptogenesis (Cheng et al., 2003; Llorens-Martín et al., 2010; Mir et al., 2017; Ransome and Hannan, 2013; Glasper et al., 2010), and shows neuroprotective features following nerve injury (Kim et al., 2021) or stroke (Zhang et al., 2013). The IGF-1 expression in the brain was decreased throughout the lifespan in the aging rats (Frutos et al., 2007), contributing to a greater susceptibility to oxidative stress (Holzenberger et al., 2003) and increased microglial activity inducing inflammation (Kohman et al., 2012).
Although muscle-derived IGF-1 is not detected in circulation, it is considered as another representative myokine. An increase in serum IGF-1 levels was observed during break periods in exercising (Ransome and Hannan, 2013). It was established that exercise-induced IGF-1 release occurs mainly after strength and resistance training (Li et al., 2022; Feng et al., 2022). IGF-1 was established to partake in anabolic processes, activate myogenesis (Feng et al., 2022), stimulate protein synthesis, and promote muscle hypertrophy (Feng et al., 2022; Yoshida and Delafontaine, 2020). The increase in muscle density might be generated via the IGF-1R/PI3K/Akt signaling pathway (Li et al., 2022; Feng et al., 2022; Kraemer et al., 2017), the stimulation of which was proposed to alleviate skeletal muscle atrophy (Yoshida and Delafontaine, 2020). PGC-1α was found to be an important element in combating insulin resistance by affecting growth hormone and IGF-1 (Kang and Li Ji, 2012). Growth hormone-induced IGF-1 release in the pituitary gland was shown to induce angiogenesis (Norling et al., 2020) and modulate the formation of pro-BDNF, leading to an increase in BDNF in the hippocampus (Ding et al., 2006). Blocking the IGF-1 receptor significantly reversed the exercise-induced increase in the BDNF expression, suggesting that the effects of IGF-1 may be caused by interceding the conversion of pro-BDNF to BDNF (Ding et al., 2006). Furthermore, exercise-induced alterations in the expression of IGF-1 in the brain were associated with synaptic and cognitive plasticity (Ding et al., 2006), and improvement in neurogenesis and memory (Trillaud et al., 2023; Ding et al., 2006). Increased IGF-1 expression in the hippocampus was observed in voluntarily running rats (Sølvsten et al., 2018). However, other studies determined a decrease in IGF-1 in male mice subjected to forced exercise, unlike females, who showed an increase in protein expression in the hippocampus (Munive et al., 2016). Munive et al. suggested that estradiol (E2) might stimulate IGF-1 uptake. Ovariectomized, exercising female mice exhibited reduced levels of IGF-1 in the hippocampus compared to the sedentary females.
Exercise-induced improvement of memory and learning, especially in animals previously subjected to chronic stress, was associated with increased expression of IGF-1 (Ding et al., 2006), FNDC5, and BDNF (Choi et al., 2024; Jiang et al., 2014) in the hippocampus (Nakajima et al., 2010; Dief et al., 2015; Phillips and Fahimi, 2018). In the MWM test, Llorens-Martin et al. demonstrated memory impairment in mice with blocked IGF-1 receptors (Llorens-Martín et al., 2010). On the other hand, treadmill-running mice with IGF-1 receptor inhibition showed exercise-induced cell proliferation compared to the sedentary group (Glasper et al., 2010).
5 Conclusion and future research directionsExercise has been suggested as a potent and robust non-pharmacological intervention for improving cognitive function, including learning and memory. Factors produced and released by skeletal muscles, including myokines, growth factors, hormones, and cytokines are considered in mediating the beneficial influence of exercise on CNS functioning, appetite, and metabolism, thus supporting the existence of a muscle-brain crosstalk. Animal studies indicate that communication between the brain and skeletal muscles is reshaped by prolonged exercise via myokine-related signaling pathways and the consequent modulation of the expression and secretion of several myokines with neuroprotective functions. Notably, the myokine profile was determined dependent on the variety of myokine-secreting cells and the exercise regimen. Whether direct or indirect, BDNF, CTSB, IL-6, and IGF-1 have been shown to facilitate the cross-talk between the brain and muscles, but the relative contribution of each myokine to the beneficial effects of exercise on the CNS remains complex.
Considering that the concept of myokines is a relatively new direction in studies covering the molecular background of exercise, the main direction of future research conceptualizations should be to identify and describe further molecules that might be classified as this type of exerkines. Pending the analysis of the muscle-brain axis in terms of bidirectional interaction via exerkine release being its direct effect, it should not be forgotten that myokines are not molecules of muscle origin only, although the hypothesis of skeletal muscles being critical sources of myokines seems promising. A limited amount of research provides information on alterations in myokines expression in both components of the axis at once. Therefore, if possible, the future directions of experimental studies on the muscle-brain axis should involve the comparison of the myokine expression in both engaged tissues, completed with the serum levels of studied exerkines. Moreover, the interaction between the brain and muscles has been examined in diverse animal models regarding experimental studies. If unified, could be a promising measure of stating more precise conclusions in terms of molecular mechanisms underlying the crosstalk and addressing the broad population. The division of specific types of training applied should be emphasized as well. Recent studies determined that the outcomes of myokines release are dependent on the type of either aerobic or resistance training that animals were exposed to. Since the muscle-brain interaction is not solely existent, interactions based on other types of exerkines are also involved in the crosstalk functionality. Particular attention should be paid to the effect of myokines on adipose tissue. Given the beneficial effects of exercise on adipose tissue remodeling, research efforts should be placed on the identification of novel exercise-induced myokines that may control adipose tissue metabolism and function. Not only do myokines partake in exercise-induced increased metabolism but also, the communication between myokines and adipokines have been found significant in improving cognition.
The terms of exercise and contingent myokines release should be drawn to researchers’ attention, given the facility of incorporating exercise as an alternative approach to decreasing the risk of CNS-linked disorders encourages further exploration.
Author contributionsMK: Investigation, Visualization, Writing–original draft, Writing–review and editing. JM: Investigation, Writing–review and editing. AM: Writing–review and editing, Supervision. MN-C: Conceptualization, Supervision, Writing–review and editing, Project administration.
FundingThe author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This work was supported by the statutory grant: AWF/NF/ZB1/2024/from the Academy of Physical Education, Katowice, Poland and from Polish National Science Center (NCN) under Grant 2022/47/B/NZ7/02135.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAbdulghani A., Poghosyan M., Mehren A., Philipsen A., Anderzhanova E. (2023). Neuroplasticity to autophagy cross-talk in a therapeutic effect of physical exercises and irisin in ADHD. Front. Mol. Neurosci. 15, 997054. doi:10.3389/fnmol.2022.997054
PubMed Abstract | CrossRef Full Text | Google Scholar
Ahsan M., Garneau L., Aguer C. (2022). The bidirectional relationship between AMPK pathway activation and myokine secretion in skeletal muscle: how it affects energy metabolism. Front. physiology 13, 1040809. doi:10.3389/fphys.2022.1040809
PubMed Abstract | CrossRef Full Text | Google Scholar
Angulo J., El Assar M., Álvarez-Bustos A., Rodríguez-Mañas L. (2020). Physical activity and exercise: strategies to manage frailty. Redox Biol. 35, 101513. doi:10.1016/j.redox.2020.101513
PubMed Abstract | CrossRef Full Text | Google Scholar
Araujo T. F., Cordeiro A. V., Vasconcelos D. A. A., Vitzel K. F., Silva V. R. R. (2018). The role of cathepsin B in autophagy during obesity: a systematic review. Life Sci. 209, 274–281. doi:10.1016/j.lfs.2018.08.024
PubMed Abstract | CrossRef Full Text | Google Scholar
Ataka K., Asakawa A., Iwai H., Kato I. (2023). Musclin prevents depression-like behavior in male mice by activating urocortin 2 signaling in the hypothalamus. Front. Endocrinol. 14, 1288282. doi:10.3389/fendo.2023.1288282
PubMed Abstract | CrossRef Full Text | Google Scholar
Augusto V., Padovani C. R., Eduardo G., Campos R. (2004). Skeletal muscle fiber types in C57BL6J mice. J. Morphol. Sci. 21 (2), 89–94.
Bai X., Yilin C., Qi X., Cai D. (2014). Single-cell analysis for BDNF and TrkB receptors in cardiac microvascular endothelial cells. Bio-medical Mater. Eng. 24 (6), 2257–2264. doi:10.3233/BME-141038
PubMed Abstract | CrossRef Full Text | Google Scholar
Baier P. C., May U., Scheller J., Rose-John S., Schiffelholz T. (2009). Impaired hippocampus-dependent and -independent learning in IL-6 deficient mice. Behav. brain Res. 200 (1), 192–196. doi:10.1016/j.bbr.2009.01.013
PubMed Abstract | CrossRef Full Text | Google Scholar
Banks W. A., Kastin A. J., Gutierrez E. G. (1994). Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci. Lett. 179 (1-2), 53–56. doi:10.1016/0304-3940(94)90933-4
PubMed Abstract | CrossRef Full Text | Google Scholar
Bechara R. G., Kelly Á. M. (2013). Exercise improves object recognition memory and induces BDNF expression and cell proliferation in cognitively enriched rats. Behav. brain Res. 245, 96–100. doi:10.1016/j.bbr.2013.02.018
PubMed Abstract | CrossRef Full Text | Google Scholar
Blume G. R., Royes L. F. F. (2024). Peripheral to brain and hippocampus crosstalk induced by exercise mediates cognitive and structural hippocampal adaptations. Life Sci. 352, 122799. doi:10.1016/j.lfs.2024.122799
PubMed Abstract | CrossRef Full Text | Google Scholar
Boström P., Wu J., Jedrychowski M. P., Korde A., Ye L., Lo J. C., et al. (2012). A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481 (7382), 463–468. doi:10.1038/nature10777
PubMed Abstract | CrossRef Full Text | Google Scholar
Burtscher J., Millet G. P., Place N., Kayser B., Zanou N. (2021). The muscle-brain Axis and neurodegenerative diseases: the key role of mitochondria in exercise-induced neuroprotection. Int. J. Mol. Sci. 22 (12), 6479. doi:10.3390/ijms22126479
PubMed Abstract | CrossRef Full Text | Google Scholar
Calvo J. A., Daniels T. G., Wang X., Paul A., Lin J., Spiegelman B. M., et al. (2008). Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J. Appl. physiology (Bethesda, Md. 1985) 104 (5), 1304–1312. doi:10.1152/japplphysiol.01231.2007
PubMed Abstract | CrossRef Full Text | Google Scholar
Carey A. L., Steinberg G. R., Macaulay S. L., Thomas W. G., Holmes A. G., Ramm G., et al. (2006). Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55 (10), 2688–2697. doi:10.2337/db05-1404
PubMed Abstract | CrossRef Full Text | Google Scholar
Chan W. S., Ng C. F., Pang B. P. S., Hang M., Tse M. C. L., Iu E. C. Y., et al. (2024). Exercise-induced BDNF promotes PPARδ-dependent reprogramming of lipid metabolism in skeletal muscle during exercise recovery. Sci. Signal. 17 (828), eadh2783. doi:10.1126/scisignal.adh2783
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen L., Zhou C., Tan C., Wang F., Gao Y., Huang C., et al. (2017). Stereological study on the positive effect of running exercise on the capillaries in the Hippocampus in a depression model. Front. Neuroanat. 11, 93. doi:10.3389/fnana.2017.00093
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen W., Wang L., You W., Shan T. (2021). Myokines mediate the cross talk between skeletal muscle and other organs. J. Cell. physiology 236 (4), 2393–2412. doi:10.1002/jcp.30033
CrossRef Full Text | Google Scholar
Chen X., Ren X., Zhu Y., Fan Z., Zhang L., Liu Z., et al. (2021). Cathepsin B-activated fluorescent and photoacoustic imaging of tumor. Anal. Chem. 93 (27), 9304–9308. doi:10.1021/acs.analchem.1c02145
PubMed Abstract | CrossRef Full Text | Google Scholar
Cheng C. M., Mervis R. F., Niu S. L., Salem N., Witters L. A., Tseng V., et al. (2003). Insulin-like growth factor 1 is essential for normal dendritic growth. J. Neurosci. Res. 73 (1), 1–9. doi:10.1002/jnr.10634
PubMed Abstract | CrossRef Full Text | Google Scholar
Chevrel G., Hohlfeld R., Sendtner M. (2006). The role of neurotrophins in muscle under physiological and pathological conditions. Muscle and nerve 33 (4), 462–476. doi:10.1002/mus.20444
PubMed Abstract | CrossRef Full Text | Google Scholar
Cho K., Yoon S. Y., Choi J. E., Kang H. J., Jang H. Y., Kim D. H. (2013). CA-074Me, a cathepsin B inhibitor, decreases APP accumulation and protects primary rat cortical neurons treated with okadaic acid. Neurosci. Lett. 548, 222–227. doi:10.1016/j.neulet.2013.05.056
PubMed Abstract | CrossRef Full Text | Google Scholar
Choi J. W., Jo S. W., Kim D. E., Paik I. Y., Balakrishnan R. (2024). Aerobic exercise attenuates LPS-induced cognitive dysfunction by reducing oxidative stress, glial activation, and neuroinflammation. Redox Biol. 71, 103101. doi:10.1016/j.redox.2024.103101
Comments (0)