The visual system is one of the most important senses, contributing to the defense and survival of animals, especially humans, and playing a crucial role in daily activities and complex behaviors (Potier et al., 2018). Visual loss tremendously impacts quality of life, affecting work and leisure activities, such as reading, driving, and watching television. Further, visual deficits/blindness are associated with psychological and social well-being, increasing the probability of developing anxiety and depression (Bradley and Delaffon, 2020).
The external part of the eyeball is composed of the cornea and sclera, followed by the iris, pupil, crystalline, and ciliary body; the posterior part comprises the retina and the choroid (Irsch and Guyton, 2009; Kels et al., 2015). Although all these components are essential to vision, the majority of non-prevented, untreatable visual deficits or blindness results from retinal pathologies (GBD 2019 Blindness and Vision Impairment Collaborators, 2021).
The Transient Receptor Potential (TRP) is an evolutionarily conserved group of channels found in invertebrates, such as the phylum nematodes and arthropods, and in vertebrates, such as fish and mammals (Vriens et al., 2004). They form ion-permeable transmembrane polymodal receptors, classically described in somatosensory neurons but also found in ocular structures, and they are sensitive to different stimulus types (Ma et al., 2022; Samanta et al., 2018). Accordingly, they can be modulated by thermal stimuli, plasma membrane stretching, endogenous/exogenous chemical molecules, fragments of pathogens, and inflammatory signals (Gilliam and Wensel, 2011). The majority of TRPs is permeable to Ca2+, a well-known second messenger, which induces several cellular responses (Reinach et al., 2015; Vangeel and Voets, 2019). Currently, seven subfamilies of TRPs are known: Ankyrin (TRPA), Canonical (TRPC), Melastatin (TRPM), Mucolipin (TRPML), NO-mechano-potential C (TRPN), Polycystic (TRPP) and Vanilloid (TRPV) (Samanta et al., 2018). TRPN was not included in this review as it has not been described in the retina.
Since the discovery of these channels, experimental studies seek the structural detail of each subfamily to reach a greater understanding of the modulatory mechanisms, as well as the action of TRP antagonists and agonists in the respective binding sites or through indirect stimulation, to broaden the understanding of the roles of this superfamily in a physiological and pathological context. In the present review, we focus on shedding light on information about the expression, modulation, and physiological function of TRPs and their putative involvement in the main human retinal pathologies.
2 Search methodThe present review searched for published studies on PubMed, Mendeley, and Scopus platforms, using keywords related to the subject topics. In the introductory topic, the combination of terms used was: “TRP” (AND) “brain” (129 articles), OR “vision” (102 articles), OR “structure” (171 articles). For all other topics, the terms “TRPC OR TRPV OR TRPM OR TRPA1 OR TRPML OR TRPP” were combined (AND) with a specific keyword related to the topic, as follows:
• Structure, functioning, exogenous/endogenous modulators, and signaling pathways: “structure OR modulation OR signaling.”
• Expression and functions in ocular structures: “Retina and retinal OR optic nerve OR pigment epithelium.”
• Ocular diseases: “Macular degeneration.” “Diabetic retinopathy,” “retina,” “methylglyoxal,” “glaucoma,” “intraocular pressure,” “retina” “retinitis pigmentosa,” “rd10,” “rd1.”
For this review, we highlighted studies published in English in the last 5 years that brought new information to the subject up to publication. However, some reviews and data published 10 years ago or more were also included. This was necessary to contextualize information about TRP structure and signal/function in retinal physiology or retinopathies. Supplementary material consists of a diagram of search strategies and the total number of articles for each one.
3 TRPs: from modulators to functioningThe first description of the TRP channels was in the Drosophila melanogaster, where abnormal retinal responses to high luminosity were documented (Reinach et al., 2015; Samanta et al., 2018). Since then, there has been an increase in research demonstrating the involvement of TRPs in retinal physiology and pathologies that lead to loss or partial impairment of vision in models of glaucoma (de Araújo et al., 2020; Choi et al., 2015; Irnaten et al., 2020; Oda et al., 2020), diabetic retinopathy (Sachdeva et al., 2018), retinitis pigmentosa (Nelidova et al., 2020), and retinal dysfunction promoted in non-ocular diseases such as mucolipidosis type IV (ML-IV) (Grishchuk et al., 2016; Venugopal et al., 2007).
All TRP subfamilies show some structural similarities. They are tetramers, where each monomer consists of six transmembrane domains (TM) (S1-S6) (Bae et al., 2018; Huang et al., 2020; Huffer et al., 2020). S5 and S6 (Huffer et al., 2020) are connected to the p-loop (Bae et al., 2018; Huang et al., 2020), and the three structures comprise the channel selectivity filter (Bae et al., 2018; de Baaij et al., 2014; Huang et al., 2020).
TRPs are a remarkably diverse group of proteins with several characteristics, which will be highlighted in the present review. Most TRP channels are permeable to monovalent and divalent ions, except for TRPV5 and TRPV6, which are highly ion-selective and have the response amplitude dependent on the extracellular Ca2+ concentration (10 and 30 mM, respectively) (Hoenderop et al., 2003; Nilius et al., 2001; Montell, 2005; Voets et al., 2001; Figure 1A). There is also an extension originating right after TM at the end of the N-terminal, called the TRP box, whose activity is related to channel activation and inactivation (Feng, 2017; Huffer et al., 2020).
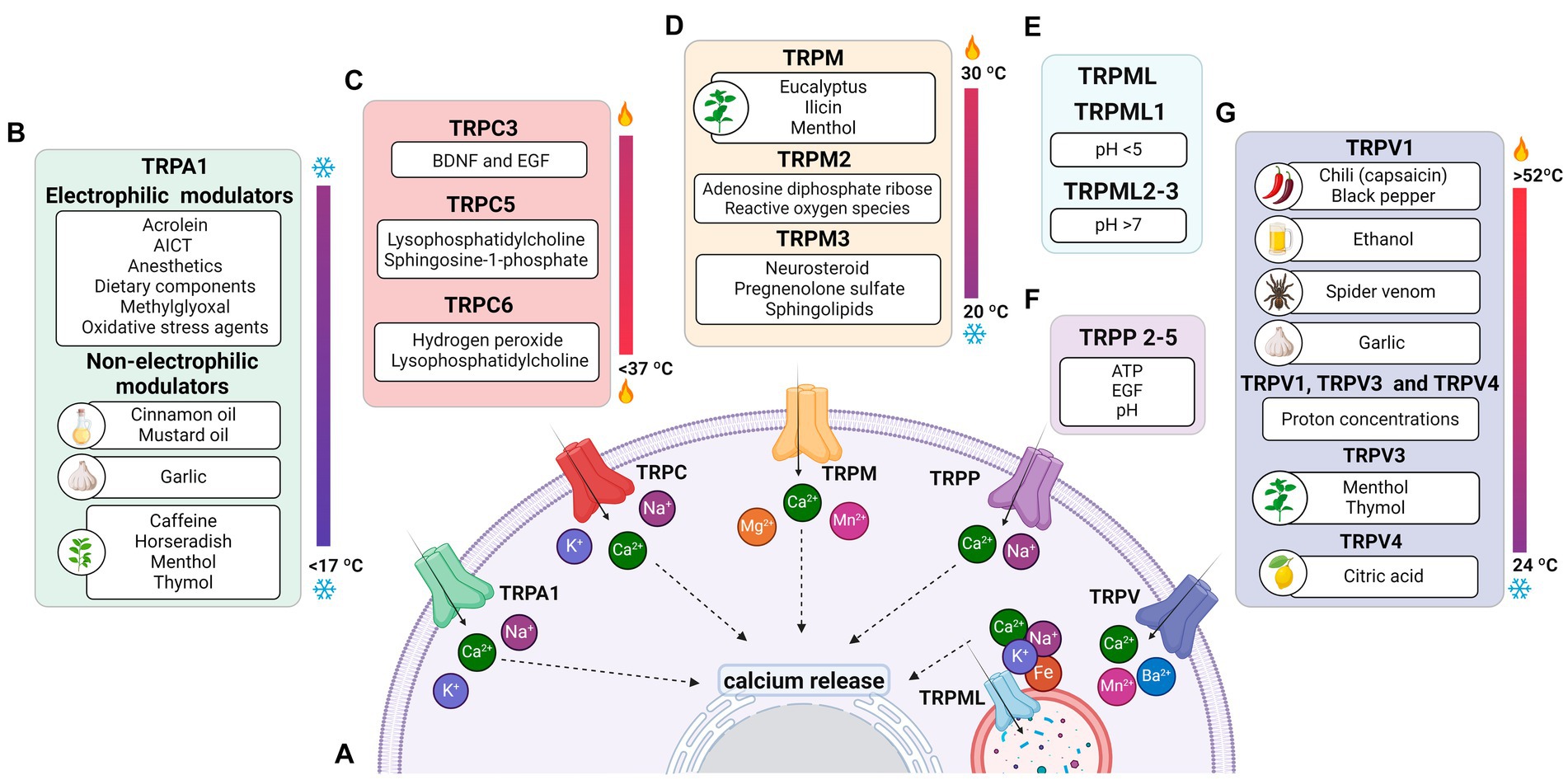
Figure 1. Permeability of TRP receptors and exogenous modulators. (A) TRPA1 receptors and TRPCs are permeable to potassium (K+), sodium (Na+), and calcium (Ca2+) ions; TRPM, TRPP and TRPVs receptors are permeable to calcium; the TRPML receptor is permeable to calcium, sodium (Na+), iron (Fe), and potassium (K+) ions. Since all receptors are permeable to calcium, their activation contributes to an increase in intracellular calcium levels and the release of ions stored in the endoplasmic reticulum. (B) Modulators of TRPA1 receptors, including acrylamide-induced cytotoxicity (AICT), dietary components, and oxidative stress agents. (C) Regulators of TRPC receptors, including brain-derived neurotrophic factor (BDNF) and epidermal growth factor (EGF). (D) Extrinsic modulators of TRPM receptors. (E) Extrinsic modulators of TRPML receptors, including adenosine triphosphate (ATP) and changes in acidity/alkalinity (pH). (F) Regulators of TRPP receptors. (G) Modulators of TRPV receptors.
The C- and N-termini constitute the cytoplasmic region of TRPs (Huffer et al., 2020). At the C-terminus of the TRPC, TRPM, and TRPA1 subfamilies, the coiled-coil (CC) domain appears to play a role in ligand anchoring and assembly of these channels in the lipid bilayer (Feng, 2017; Hilton et al., 2019; Nooren et al., 1999). Furthermore, the N-terminus of TRPA1, TRPC, TRPV, and TRPM exhibits repeats of the ankyrin protein, whose function is related to conformational changes of the ionic pore and target of specific molecules in some TRPs (Feng, 2017; Hilton et al., 2019; Li et al., 2019).
The central pore and the TRP box are highly conserved in the different TRP subfamilies, while S1-S4 show structural modifications, and the C-terminus and N-terminus are quite variable between subfamilies. These differences contribute to establishing specificities and modulatory mechanisms in TRP channels (Huffer et al., 2020; Li et al., 2011). Similarities and differences in the structural components between TRP subfamily members, modulating molecules, as well as the response after activation of these channels, are summarized in Table 1.
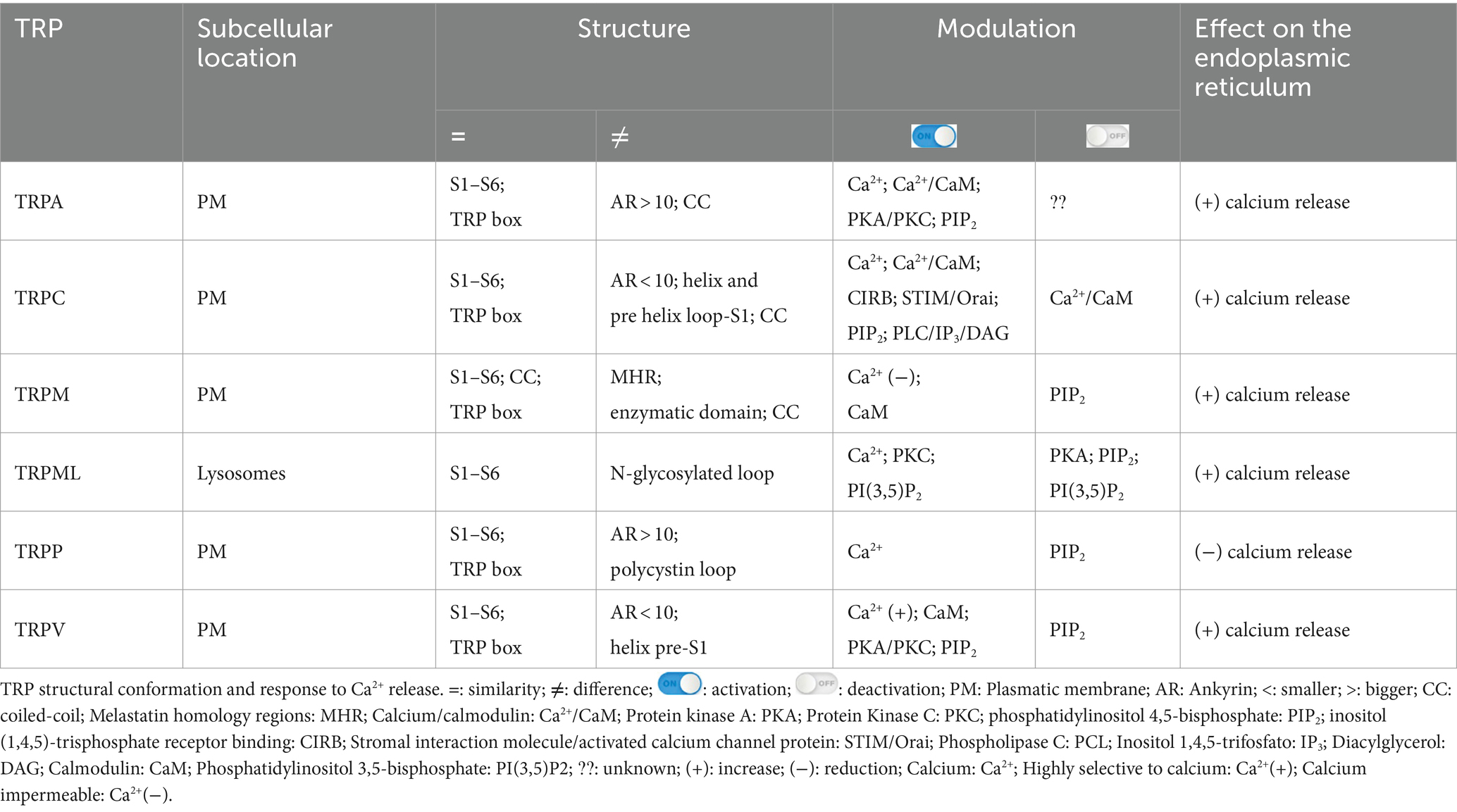
Table 1. Structural components, modulatory and response mechanism peculiarities among all TRP subfamilies.
3.1 TRPA1TRPA1 is a cation-permeable channel, especially to Ca2+ (Talavera et al., 2020; Figure 1A). This receptor is mechanical-, thermo-, and chemo-sensitive and can be activated by exogenous and endogenous agents (Kádková et al., 2017). There are binding sites for channel-modulating substances in TRPA1 transmembrane domains, which also appear important for voltage-sensitivity and -dependence. Marsakova and collaborators suggest a binding site for Ca2+ in the external region of the TRPA1 protein and that the S1-S2 domains are physically coupled to the gating regions of the channel (Marsakova et al., 2017). On the other hand, recent experiments by Zhao et al. (2020) suggest that Ca2+-dependent modulations appear to be concentrated in S2–S3 (Zhao et al., 2020). The size of the NH2-terminal is equivalent to all the rest of the protein, consisting of 14–18 repetitions of ankyrin, a characteristic that gave TRPA1 its name (Meents et al., 2017; Nilius et al., 2011). In addition to being a binding site for several molecules, such as cytosolic Ca2+, it also appears to participate in thermal and chemical responsiveness (Cordero-Morales et al., 2011; Jabba et al., 2014).
The N-terminal region can regulate and integrate stimuli (Zayats et al., 2013; Hynkova et al., 2016). Many possible forms of channel modulation involving the N-terminal region have been described: (1) irritating agonists and electrophiles (isocyanates, cinnamaldehyde, garlic diallyl disulfide, acrolein) can chemically react with primary amines and cysteines (Bahia et al., 2016; Suo et al., 2020) inducing channel opening (Hinman et al., 2006; Macpherson et al., 2007); (2) direct calcium binding participating in desensitization (Cordero-Morales et al., 2011); (3) direct interaction with proteins such as FGFR2, which inhibit TRPA1 (Berrout et al., 2017); (4) phosphorylation, for example by protein kinase A (PKA) or protein kinase C (PKC), which can increase levels of the channel on the plasma membrane. The C-terminal termination also has Ca2+ binding sites but seems more associated with voltage-dependent responses (Sura et al., 2012). A summary of the stimuli of TRP is shown in Table 2.
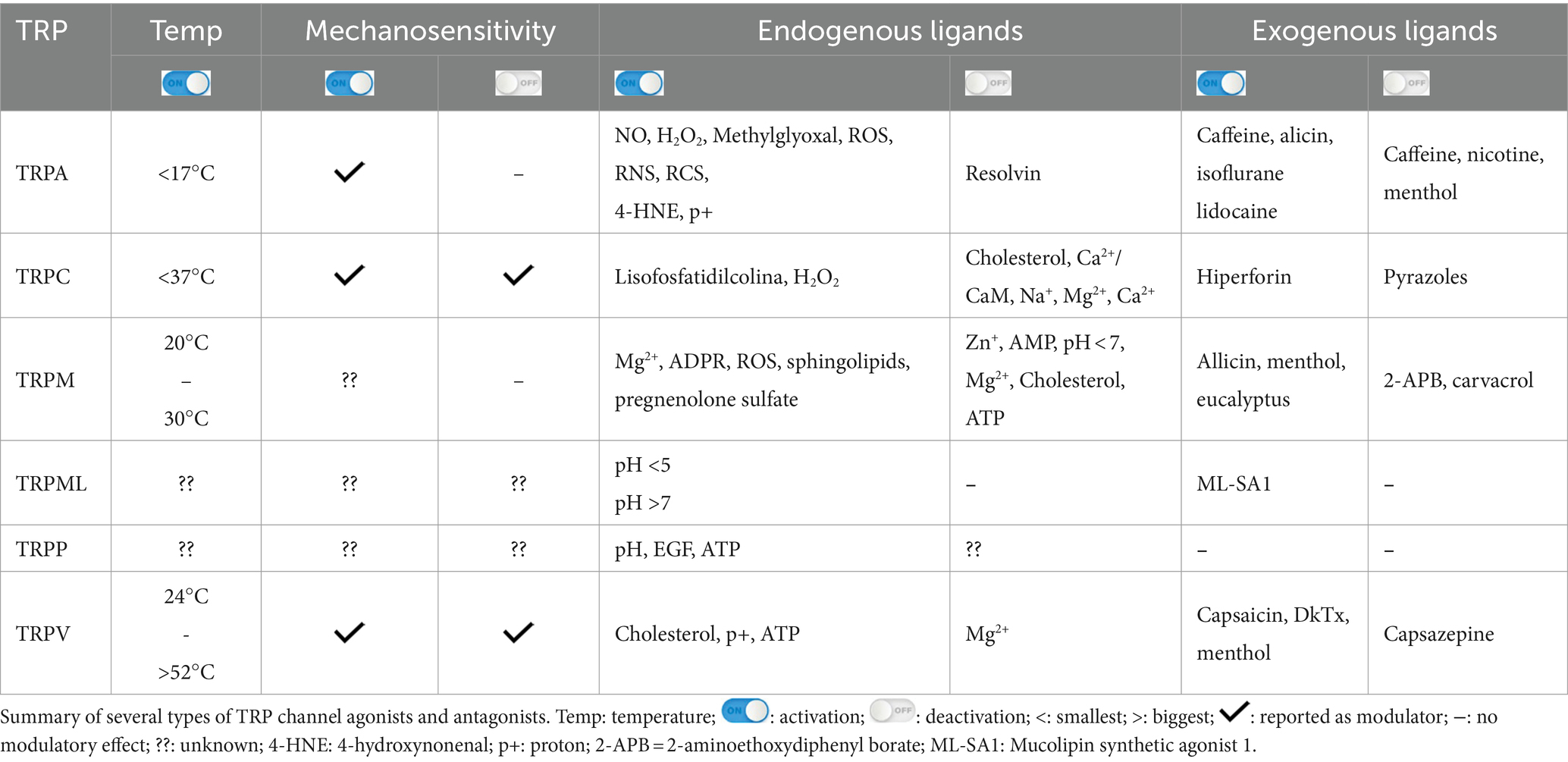
Table 2. Modulators of TRP channel activity.
It is known that the activation of all TRPs promotes the entry of Ca2+ into the cell, which, in turn, triggers the activation of biochemical pathways, such as the calcium-dependent-phospholipase C (PLC) pathway (Kouchi et al., 2004), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trifosfato (IP3) and diacylglycerol (DAG) (Wang et al., 2020) and then IP3 binds to IP3 receptors located in the endoplasmic reticulum (ER), promoting a transient increase in cytosolic Ca2+ (Curcic et al., 2019; Mori et al., 2015; Figure 1A). PIP2 is a major modulator of TRP channels, and it can sometimes inhibit or activate, as will be discussed later (Pumroy et al., 2020). Usually, one of the interaction sites with PIP2 is located at the N-terminus of TRPs, and in the case of TRPA1, it is also located at the C-terminus (Macikova et al., 2019).
TRPA1 can be modulated by a huge variety of chemical compounds, divided into electrophilic and non-electrophilic modulators, according to the ability of each group to interact covalently or noncovalently, respectively (Talavera et al., 2020; Figure 1B). Among the former group, there are dietary components such as cinnamaldehyde, allicin (Bandell et al., 2004), isothiocyanates (Bandell et al., 2004), thymol, methyl salicylate; anesthetics as isoflurane, lidocaine, and propofol (Matta et al., 2008); and oxidative stress agents such as nitric oxide (NO), hydrogen peroxide (H2O2), and methylglyoxal (MG) (Cao et al., 2012; Meents et al., 2017), although reactive oxygen (ROS), nitrogen (RNS), and carbonyl (RCS) species are all, in general, potential activators (Bessac and Jordt, 2008; Nilius et al., 2011). In the context of oxidative stress, the product of lipid peroxidation, 4-hydroxynonenal (4-HNE), is also a potent activator of TRPA1 (Trevisani et al., 2007). The non-electrophilic group includes some commonly consumed components, such as menthol (Karashima et al., 2009), thymol (Lee et al., 2008), carvacrol (Xu et al., 2006), nicotine, caffeine, and Δ9–tetrahydrocannabinol; medication like nifedipine and nonsteroidal anti-inflammatory drugs (Jordt et al., 2004; Nassini et al., 2015; Talavera et al., 2009); and protons (Nilius et al., 2011). Interestingly, some ligands have a bimodal effect, activating the channel in low concentrations while inhibiting it at higher doses, as it is the case of nicotine and menthol (Karashima et al., 2009; Talavera et al., 2009). Furthermore, there are also components with species-specific effects when it comes to modulation of the channel; for example, caffeine can activate TRPA1 in mice but suppresses it in humans (Nagatomo and Kubo, 2008).
Ca2+ is another important modulator of TRPA1 activity. It interacts intracellularly first to activate and then increases the response to other activators, followed by an inactivation process (Sura et al., 2012). Both potentiation and inactivation appear to be mediated by independent processes (Wang et al., 2008). In addition, there is evidence that modulation by Ca2+ is dependent on the direct binding of calmodulin to the channel, which affects both the activation and inactivation steps (Hasan et al., 2017), although the direct action of Ca2+ on human TRPA1 has also been demonstrated by calmodulin-independent mechanisms (Moparthi et al., 2020).
Protons can also activate TRPA1 in the context of acidosis, a common event in ischemia, for example. Intracellular protons are more commonly accepted as modulators, while extracellular activation is species specific (de la Roche et al., 2013). Additionally, phosphorylation sites for kinases, such as PKA and cyclin-dependent kinase 5 (Cdk5), have been identified and correlated with the capacity to promote sensitization of the channel, enabling increased responsiveness to agonist stimulation (Meents et al., 2017; Hall et al., 2018). As demonstrated recently, PKC can also sensitize the channel (McMillan et al., 2021), but data concerning more direct interaction needs further analysis.
3.2 TRPCTRPC 1–7 are classified as non-selective channels, permeable to Na+, K+, and Ca2+ (DeHaven et al., 2009; Figure 1A). TRPCs are abundantly expressed in immune cells, endothelium, heart, striated muscle, pancreas, kidneys, placenta, retina, pituitary gland, cerebellum, limbic system, and substantia nigra (Wang et al., 2020; Warren et al., 2006). Only TRPC2 is not expressed in human cells (de Souza and Ambudkar, 2014).
The carboxy-terminal region commonly comprises domains that bind Ca2+/CaM complex and inositol (1,4,5)-trisphosphate receptor binding (CIRB), and other molecules, such as trimeric G proteins (Friedlova et al., 2010; Wang et al., 2010; Wang et al., 2020). Although the mechanisms of TRPC activation are not yet well described (Wang et al., 2020), it is known that they are channels dependent on PLC, either activated by G protein-coupled receptors or tyrosine kinase receptors (Feng, 2017; Li et al., 2019; Wang et al., 2020; Zhu, 2005).
In response to PLC signaling, there is an elevation in calcium levels in the cytoplasm, with a consequent depletion of Ca2+ stores in the ER, resulting in activation of TRPC, which promotes robust and sustained Ca2+ entry by the channel (Curcic et al., 2019; Hofmann et al., 1999; Mori et al., 2015; Zhu, 2005).
Therefore, it is proposed that TRPC represent store-operated Ca2+ entry channels (SOCE), as they play a key role in increasing cytoplasmic Ca2+ (DeHaven et al., 2009; Feng, 2017; Li et al., 2019). Furthermore, intracellular Ca2+ moderately potentiates TRPC4 responses and considerably TRPC5, possibly due to a longer opening time of both ionic pores (Blair et al., 2009; Mori et al., 2015). Although TRPC1 alone functions as a SOCE in human adult retinal pigment epithelial-19 (ARPE-19) cells (Bollimuntha et al., 2005), TRPC3 and 4 only function as SOCE when co-expressed with TRPC1 (de Souza and Ambudkar, 2014). It has already been demonstrated that these TRPCs function as a SOCE channel in cultured Müller glia and 129/SvImJ mice (Molnár et al., 2016; Da Silva et al., 2008).
Physical stimuli have been linked to the activation of TRPCs. TRPC1, C5, and C6 are characterized as mechanosensitive receptors (Choi et al., 2015; Irnaten et al., 2020), while TRPC5 is also stimulated by temperatures below 37°C (Oda et al., 2020; Figure 1C).
TRPC1, 4, and 5 are activated directly by phospholipase C (Duan et al., 2018; Chen et al., 2020). Both TRPC3 and C5 can be stimulated by IP3, while C3, 6, and 7 exhibit varying sensitivity in the presence of PIP2 (Wang et al., 2020). TRPC2, 3, 6, and 7 are directly stimulated by DAG (Fan et al., 2018; Li et al., 2019; Wang et al., 2020), while TRPC may not be stimulated by DAG in ipRGCs culture (Graham et al., 2008). Although TRPC4 and C5 are not activated by DAG, they may become sensitive to the molecule and its derivatives (Curcic et al., 2019; Storch et al., 2017). This sensitivity change in TRPC4-5 occurs while PKC activity is inhibited since the kinase deactivates these TRPCs as well as PIP2 (Storch et al., 2017).
3.3 TRPMThe TRPM family consists of eight members (TRPM1–TRPM8), expressed in cells distributed in different tissues, such as the retina, kidney, pancreas, prostate, heart, intestine, and immune system (Huang et al., 2020; Sawamura et al., 2017). In the nervous system, TRPM is detected in neurons and glial cells (astrocytes, oligodendrocytes, and microglia) (Sawamura et al., 2017).
TRPM1, TRPM2, TRPM3, TRPM6, TRPM7, and TRPM8 share similarities in generating responses through Ca2+ influx (Bae et al., 2018), while TRPM4 and TRPM5 are voltage-dependent and Ca2+ impermeable (Huang et al., 2020; Samanta et al., 2018). TRPMs are also permeable to Mg2+ and Mn2+; Mg2+ negatively modulates the TRPM1 channel expressed in the photoreceptor (Jimenez et al., 2020; Figure 1A). In addition, TRPM5 is activated by intracellular Ca2+ with greater sensitivity than TRPM4 (Ruan et al., 2021; Zierler et al., 2017).
The TRPM has particular structural characteristics that are distinct from other TRPs. They are the receptors with the highest amount of amino acid residues in the cytosolic region and do not have the N-terminal ankyrin repeats (Huang et al., 2020; Kraft and Harteneck, 2005; Samanta et al., 2018). The N-terminus comprises four melastatin homology regions (MHRs), while the C-terminus exposes two domains (Chen et al., 2019; Guo et al., 2017).
Furthermore, the cytoplasmic domain may show differences between members of the TRPM family (Huang et al., 2020; Jirku et al., 2015; Zierler et al., 2017). For example, most members of the TRPM family, including TRPM1, exhibit binding sites in both the transmembrane domain and the N-terminus for interaction with PIP2 (Huang et al., 2020; Jirku et al., 2015; Zierler et al., 2017). The C-terminus of TRPM2, 6, and 7 have enzymatic domains (Guo et al., 2017; Jirku et al., 2015; Samanta et al., 2018). The enzymatic domains reported in experimental data at the C-terminus of TRPM6 and 7 are comprised of alpha kinases, a class of kinases that preferentially phosphorylate on the alpha helix of the target molecule. These kinases can induce positive modulatory functions related to Mg2+ (Cabezas-Bratesco et al., 2015; Cai et al., 2017); the ion also plays a negative role in the activation of TRPM1 in retinal cells (Rampino and Nawy, 2011). Conditions of increased ROS production and oxidative stress induce ADPR release and TRPM2 activation (Tóth et al., 2014; Huang et al., 2019; Huang et al., 2018; Figure 1D).
It has been described that at the N-terminus of TRPM2, there is an isoleucine glutamine (IQ) domain, reported as the main binding site for the Ca2+/CaM complex (Du et al., 2009; Kim et al., 2008; Syed Mortadza et al., 2015). Experiments involving mutations in amino acids of the IQ motif resulted in inactivity of TRPM2 when the channel was stimulated by increased intracellular Ca2+ or ADPR, suggesting that the Ca2+/CaM complex plays a crucial role in the activation of TRPM2 (Du et al., 2009).
TRPMs play a variety of physiological and biochemical roles in health and disease (Jimenez et al., 2020). For example, TRPM1 plays a key role in the response of retinal ON-bipolar cells (Rampino and Nawy, 2011; Wong et al., 2019); TRPM2 is an oxidative stress sensor in human monocytic cells (Belrose et al., 2012; Sawamura et al., 2017; Wehrhahn et al., 2010); TRPM3, TRPM4, and TRPM5 are involved in the recognition of high temperatures, and TRPM8 in cold (Talavera et al., 2005; Vriens et al., 2011; Figure 1D). In addition, this channel has also been classically described to be activated by organic compounds such as menthol, eucalyptus, and ilicin (Jimenez et al., 2020; Kraft and Harteneck, 2005; Venkatachalam and Montell, 2007; Figure 1D). TRPM6 and TRPM7 play a role in Mg2+ homeostasis (Chen et al., 2019; Jin et al., 2008; Wong et al., 2019).
TRPMs are also stimulated by a variety of endogenous agents: TRPM2 activation occurs by ADPR and its analogs, in addition to reactive oxygen species (ROS) (Jimenez et al., 2020; Kraft and Harteneck, 2005; Montell, 2005); TRPM3 is activated by sphingolipids, a class of membrane lipids (Jimenez et al., 2020; Kraft and Harteneck, 2005), and neurosteroid pregnenolone sulfate (PS) during the development of mice retinal cells (Samanta et al., 2018; Shiels, 2020); both TRPM4 and TRPM5 are activated by increased intracellular Ca2+ and the co-expression of TRPM6 and TRPM7 turn them responsive to intracellular Mg2+ concentrations ranging from 0.3 to 1 mM (Ferioli et al., 2017; Montell, 2005; Figure 1A). TRPM5 is positively stimulated by extracellular adenosine triphosphate (ATP) (Hofmann et al., 2003; Jimenez et al., 2020), and there is evidence that TRPM7 shows the same modulation (Jimenez et al., 2020; Kraft and Harteneck, 2005; Montell, 2005).
The TRPM family is blocked by a variety of molecules and microenvironmental conditions (Jimenez et al., 2020). TRPM1 is down-regulated by Zn2+ (Lambert et al., 2011); TRPM2 is inactivated by AMP and pH < 7 (Du et al., 2009; Lange et al., 2008); TRPM3 is blocked by Mg2+ and cholesterol (Lambert et al., 2011); TRPM4 is inactivated by cytosolic ATP (Guo et al., 2017); TRPM5 is blocked by acidic pH (D. Liu et al., 2005); and TRPM6 and TRPM7 are blocked by Mg2+ (Ferioli et al., 2017). TRPM6 dependent-P2X4 inhibition has also been demonstrated (de Baaij et al., 2014), while the inactivation of TRPM8 occurs by the activity of bradykinin and histamine signaling during inflammatory processes (Zhang et al., 2012a,b).
3.4 TRPML/TRPPTRPML (1–3) are expressed in kidneys, lungs, endocrine tissues, heart, and brain (Samanta et al., 2018; Wang et al., 2014). TRPML1 is the best known and studied within the subfamily, while the other two channels have not yet been reported to impact the health or pathologies of the organism. Therefore, in relation to permeability, it is only known that TRPML1 is permeable to Ca2+, Na+, K+, Fe2+, and Zn2+ (LaPlante et al., 2002; LaPlante et al., 2004; Zhang et al., 2018; Pedersen et al., 2005; Figure 1A).
TRPML channel proteins have fewer amino acids than other TRPs, making them smaller, with no ankyrin repeats, with an incomplete TRP box, and. Binding sites for PKC between S1 and S2 (Samanta et al., 2018; Spix et al., 2020; Wang et al., 2014).
Moreover, TRPML1 participates in phagocytosis and protein trafficking (LaPlante et al., 2002; Spix et al., 2020). Importantly, TRPML1 mutations, or deletions in the channel, cause ML-IV with serious deleterious ophthalmologic symptoms, including strabismus, myopia, light sensitivity, and retinal degeneration (Smith et al., 2002; Grishchuk et al., 2016).
As illustrated in Figure 1E, variations of pH activate these channels: TRPML1 by pH <5 and TRPML2 and 3 by pH >7 (Spix et al., 2020). Similar to most TRPs, TRPML is mainly up-regulated, but in some cases down-regulated, by phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2], found in lysosomes (Dayam et al., 2015; Zhang et al., 2012a,b). The engulfment of potentially harmful particles by phagocytic cells, for example, induces the formation of PI(3,5)P2, which acts directly by stimulating TRPML1, expressed in the lumen of organelle, to evoke Ca2+ release (Dayam et al., 2015; Zhang et al., 2012a,b; Wang et al., 2014).
PKA can inhibit TRPML1, and when the channel is expressed in the plasma membrane, it is inactivated by low extracellular pH and PIP2. PIP2 inactivates TRPML1 even in the presence of the agonist, while phospholipid hydrolysis has been shown to activate the channel in HEK293 (Venkatachalam et al., 2015; Wang et al., 2014; Zhang et al., 2012a,b).
Similar to TRPML channels, there is still little information about the permeability of the TRPP subfamily, with only Ca2+permeability known for TRPP2-5 and, specifically, for P2 being permeable to Na+ as well (Pedersen et al., 2005).
Due to structural differences from TRP and other TRPPs, TRPP1 was considered to form another family known as polycystin1 (PC1), which is a non-functional channel. TRPPs are channels found in various tissues, such as the nervous system, heart, placenta, intestine, and pancreas (Hofherr and Köttgen, 2011; Samanta et al., 2018).
TRPPs have only one specific structural feature, an extensive loop called polycystin, which projects to the cytoplasm and is found in the middle of S1-S2. This is similar to TRPML, whereas in TRPP this structure is related to the stabilization of the channels in the plasma membrane (Hofherr and Köttgen, 2011; Samanta et al., 2018).
Wegierski’s group proposes that Ca2+ release in ER is reduced in the presence of TRPP2, and the absence of the channel promotes a huge outflow of Ca2+ from the organelle (Wegierski et al., 2009). Similar to TRPC, TRPP2 plays a role in balancing ER Ca2+ concentrations (Hofherr and Köttgen, 2011). On the other hand, Zheng and colleagues suggest that PIP2 has an inhibitory role in TRPP2-3 (Zheng et al., 2018). Further studies are needed for TRPP to elucidate the role of modulators. It has been mentioned that pH (Huang et al., 2006; Ng et al., 2019), EGF (Zhang et al., 2013) and ATP (Cantero Mdel et al., 2015) are activators and that natural exogenous ligands are unknown (Figure 1F).
3.5 TRPVThe TRPV subfamily comprises six channels (1–6), permeable to Ca2+, Ba2+, Mn2+ (Owsianik et al., 2006; Figure 1A). These channels are commonly expressed in the dorsal root, trigeminal, vagus nerves (Samanta et al., 2018; Seebohm and Schreiber, 2021), retina, and optic nerve (Choi et al., 2015; Leonelli et al., 2010; Leonelli et al., 2009), but also in other tissues, smooth muscle, digestive system, kidneys, and pancreas (Samanta et al., 2018; Seebohm and Schreiber, 2021).
TRPV has a smaller helix that precedes S1 and six ankyrin repeats that recognize modulatory molecules (Garcia-Elias et al., 2015; Pumroy et al., 2020). In the amino and carboxy terminal regions, there are binding sites to CaM, ATP (Phelps et al., 2010; Seebohm and Schreiber, 2021), PKA and PKC (Mamenko et al., 2013; Seebohm and Schreiber, 2021) that can regulate the activity of these channels (Mamenko et al., 2013; Niemeyer, 2005; Phelps et al., 2010). In particular, PIP2 has a controversial function in TRPs (Pumroy et al., 2020). Low levels of the phospholipid inhibit TRPV1, as well as partially inactivate V2, and completely V6, under the same conditions (Cao et al., 2013; Lukacs et al., 2007; Mercado et al., 2010). PIP2 can stimulate V1 in high concentrations of TRPV1 agonists (Lukacs et al., 2007); the reduction of PIP2 activates V3, and in V4, the phospholipid stimulates the channel during response to heat and decreases resistance to muscle stretch; V5 is activated by elevated PIP2 levels (Doerner et al., 2011; Garcia-Elias et al., 2013; Lee et al., 2005), PIP2 also has the potential to enhance TRPV3 functionality and regulate TRPV5 inhibitory responses to Mg2+, highlighting the complexity and controversial activity of inositol phospholipid (Lee et al., 2005; Pumroy et al., 2020; Seebohm and Schreiber, 2021).
Originally, most TRPV channels respond to thermal stimuli: TRPV1 (> 42°C), TRPV2 (> 52°C), TRPV3 (31–39°C), and TRPV4 (24–33°C). TRPV5 and TRPV6 are not heat sensors but playa relevant role in Ca2+ regulation, given their high ionic selectivity. The TRPV subfamily responds to elevated osmotic pressure, as seen in a glaucoma model, by activating TRPV1 (Sappington and Calkins, 2008). Additionally, it activates TRPV2 and TRPV4 (Muraki et al., 2003; Vriens et al., 2004) while inactivating TRPV5 and TRPV6 (Gangwar et al., 2017; Lewis et al., 2011; Figure 1E).
Capsaicin was the first molecule described to stimulate TRPV1 (Caterina et al., 1997; Figure 1E). Furthermore, V1 was reported to be activated by ethanol in sensory nerves, while garlic extract activated V1 channels expressed in Xenopus oocytes. The amplitude of the TRPV3 response depends on the increase in menthol concentration; the channel is also activated by thymol. TRPV4 has been reported to be stimulated by citric acid solution (Buday et al., 2017; Macpherson et al., 2005; Nicoletti et al., 2008; Vogt-Eisele et al., 2007). TRPV1 and TRPV4 detect high concentrations of protons, while TRPV3 responds to low concentrations (Seebohm and Schreiber, 2021). Furthermore, cholesterol acts on TRPV1 and TRPV3 (Klein et al., 2014; Picazo-Juárez et al., 2011).
It is possible that activation of V1-V2 promotes Ca2+ entry into the cell, which, in turn, triggers activation of a type of PLC sensitive to calcium (Lukacs et al., 2007; Mercado et al., 2010). This hydrolyzes PIP2 into IP3 and DAG (Wang et al., 2020). Then, IP3 binds to IP3 receptors located in the ER, promoting a transient increase of cytosolic Ca2+ (Curcic et al., 2019; Mori et al., 2015).
Mercado and collaborators suggest that PIP2 binds directly to active TRPV2, and the hydrolysis of the phospholipid in the plasma membrane is dependent on the entry of Ca2+ into the channel; this negative modulation of PIP2 levels attenuates the responses triggered by TRPV2 (Mercado et al., 2010). Interestingly, the Ca2+/CaM complex did not bind to the channel’s ankyrin repeats, contrary to what was observed in the other TRPVs. Mercado et al. (2010) have described that this complex might not be functional and therefore would have no role in TRPV2 desensitization (Mercado et al., 2010; Rosenbaum et al., 2004; Strotmann et al., 2003). It was later reported that deletion of the Ca2+/CaM complex binding site from the N-terminus prevented interaction with IP3 receptors, and although TRPV4 still triggered Ca2+ influx, no current amplification was observed in the presence of ATP. These results indicate that the channel activity does not depend on the Ca2+/CaM complex, but the responses are potentiated by communication with the protein binding site (Garcia-Elias et al., 2008; Niemeyer, 2005).
TRPV4 current can be significantly enhanced by cytoplasmic ATP. Interestingly, this modulation only occurs when the channel is activated by osmotic changes, but not by temperature (Fernandes et al., 2008).
4 TRPs expression and functions in the healthy retina and disease conditionsThe retina is organized into three cellular layers interspersed with two plexiform layers (Wässle, 2004). The cell layers include the ganglion cell layer (GCL), composed by cell bodies of ganglion cells and displaced amacrine cells; the inner nuclear layer (INL), constituted of bipolar, amacrine, horizontal cells and Müller cells; and the outer nuclear layer (ONL), composed by photoreceptor cells (rods and cones) (Masland, 2001). Synaptic contacts in the retina occur in the inner plexiform layer (IPL), located between the GCL and INL, and the outer plexiform layer (OPL) between INL and ONL. Therefore, the IPL contains synapses of ganglion cells, amacrine, and bipolar cells, while the OPL presents synapses between photoreceptors and bipolar and horizontal cells (Wässle, 2004). Ganglion cell axons form the optic nerve, which connects the retina to brain visual areas. The retinal pigmented epithelium (RPE) is the outermost layer of the retina, adjacent to the choroid, and is important for the physiology of the healthy retina (Wimmers and Strauss, 2007). The RPE controls the trafficking of substances by removing debris from degenerated photoreceptors, ion flux, and releases protective signals, among other functions. Pigmentation of epithelial cells plays a crucial role in preventing light scattering, important to a better resolution of image formation (Wimmers and Strauss, 2007). TRPs family members are widely distributed in the retina (Figure 2).
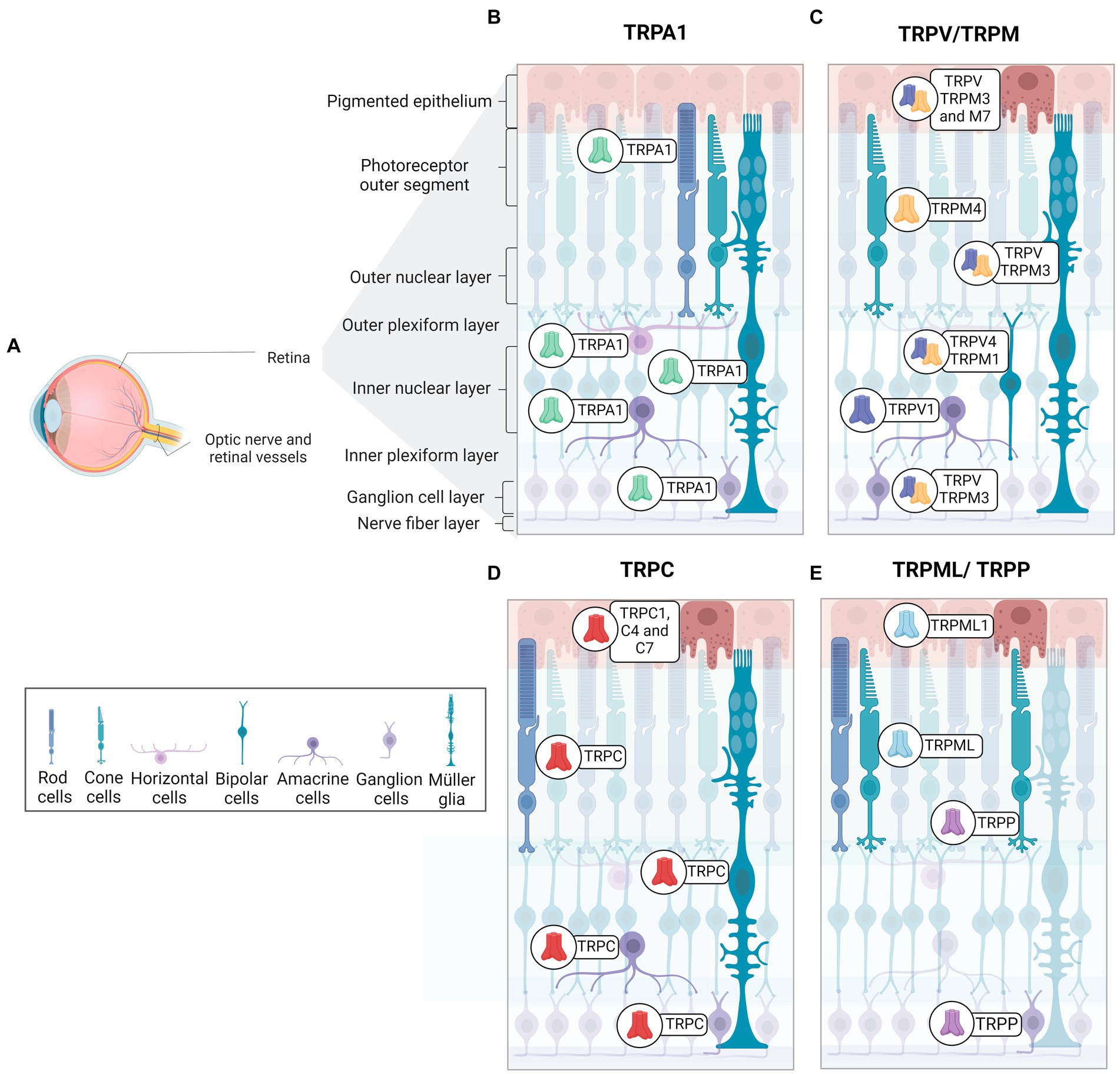
Figure 2. Distribution and expression of TRP receptors in retinal cells. (A) Schematic representation of a radial section of the eye indicating the location of the retina and optic nerve. (B) TRPA1 receptors are present in photoreceptors, horizontal cells, Müller glia, amacrine cells, and ganglion cells. (C) TRPM receptors are identified as follow in epithelial cells (TRPM2, TRPM3 and TRPM7), Müller glia and ganglion cells (TRPM3), cone-type photoreceptors (TRPM4), bipolar cells (TRPM1). (D) TRPC receptors are distributed in retinal pigmented epithelium cells (TRPC1, TRPC4, TRPC7), rod-type photoreceptors, Müller glia (TRPC1, TRPC5, TRPC6), bipolar cells (TRPC6), amacrine cells (TRPC5, TRPC6), and ganglion cells (TRPC5, TRPC6). (E)TRPML1 receptors are expressed in retinal pigment cells and photoreceptors. TRPP2 receptors are found in cone photoreceptors and ganglion cells. TRPV receptors are observed in pigment epithelium (TRPV1-TRPV4), Müller glia (TRPV1, TRPV4), amacrine cells (TRPV1), bipolar cells (TRPV4) and ganglion cells (TRPV1).
Araújo and collaborators detected TRPA1 in amacrine, horizontal, ganglion cells, Müller glia, and photoreceptors in mouse and human retinas (de Araújo et al., 2020; Figure 2B). Although TRPA1 seems to play a crucial role in the cell death promoted by ischemia (de Araújo et al., 2020; Araújo et al., 2017), the function in the healthy retina is still undescribed. The same group also demonstrated the presence of TRPA1 in the chick retina, and its role in cell death induced by acute ischemia (Araújo et al., 2017). Table 3 summarizes the expression of TRPs in regions of retina.
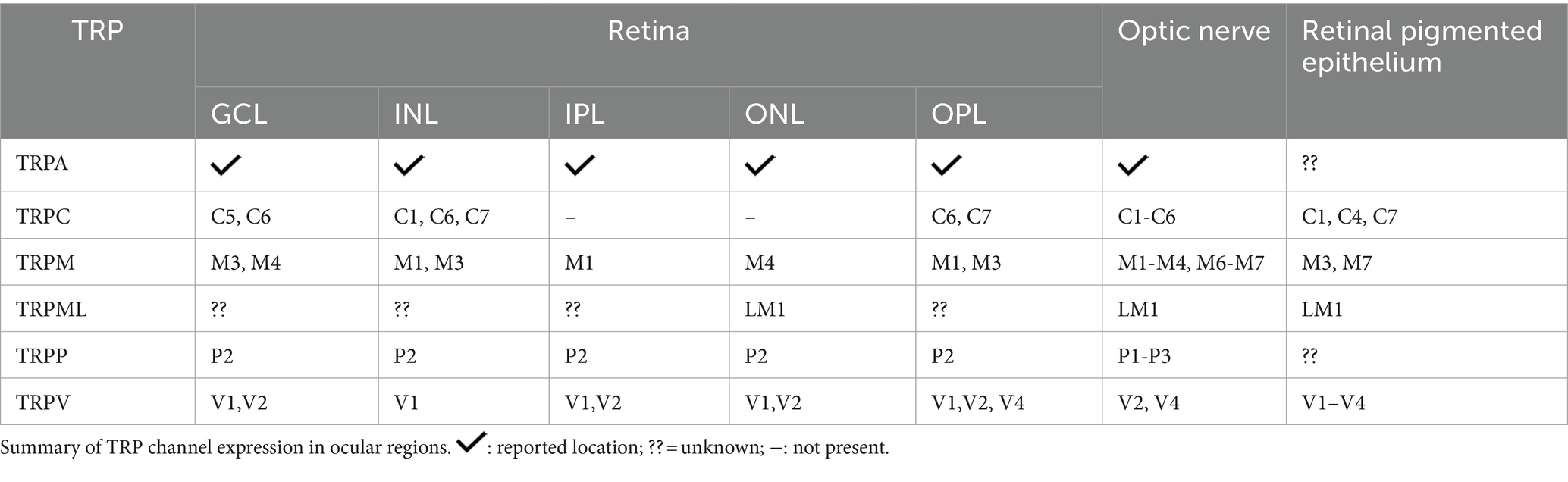
Table 3. Expression of all TRPs in the retinal layers and optic nerve
TRPC5 channels are expressed in Müller glial cells, amacrine cells, displaced amacrine, and ganglion cells of C57BL/6 mice retina (Oda et al., 2020; Wang et al., 2010; Figure 2C). Müller glia responds to various stimuli, such as mechanical stimuli, and indirectly to light, with an intense increase in cytoplasmic Ca2+ (Da Silva et al., 2008). Müller glia in vitro express TRPC1 and C6 mRNA and responds to DAG and its analog with potentiated Ca2+ current, suggesting a pivotal role for TRPC as SOCE channels in this cell (Molnár et al., 2016; Da Silva et al., 2008). In agreement, TRPC1/3 knockouts (Molnár et al., 2016) and specific pharmacological blockers of TRPC promote a marked decrease in intracellular Ca2+ currents in Müller cells, supporting the hypothesis that SOCE is a TRPC channel (Molnár et al., 2016; Da Silva et al., 2008).
TRPC6 actively participates in phototransduction in intrinsically photosensitive RGCs (ipRGCs), but C3 and C7 may have a role in the response mechanism of ipRGCs, a small group of ganglion cells that contain melanopsin and respond to light with depolarization and Ca2+ influx (Berson, 2007; Perez-Leighton et al., 2011). ipRGCs are involved with contrast control, day-night cycle, pupil diameter modulation, and humor (Berson, 2007; Contreras et al., 2021; Sonoda et al., 2018).
TRPC6 and C7 are expressed in ganglion cells, INL, and OPL (Perez-Leighton et al., 2011; Warren et al., 2006), and TRPC6 also occurs in ipRGCs (Perez-Leighton et al., 2011; Figure 2C). TRPC3, 6, and 7 channels are activated by high light intensity (Sonoda et al., 2018).
Many studies have examined phototransduction in ipRGCs. It is suggested that Gq protein activation induces PLC/PIP2 hydrolysis and generates IP3 and DAG, which stimulate TRPC and increase intracellular Ca2+ in response to light stimulation (Contreras et al., 2021; Bailes and Lucas, 2010; Graham et al., 2008). However, despite data proposing that neither DAG nor its derivatives were able to trigger action potentials in ipRGCs in an attempt to activate TRPC channels (Graham et al., 2008), other results have shown that phototransduction was blocked pharmacologically through the use of inhibitors for TRPC, which also act on IP3 (Bailes and Lucas, 2010; Sekaran et al., 2007).
TRPC4 occurs in human retinal microvascular endothelial cells (HRMECs) and it seems to be related to angiogenesis. The vascular endothelial growth factor (VEGF), the main modulator of the growth of new blood vessels, lost its angiogenic property in TRPC4 knockdown HRMECs cell culture, suggesting a role for TRPC4 as a modulator in retinal neovascularization (Song et al., 2015).
Furthermore, it was recently demonstrated that TRPC5 inhibition induces axon elongation through the optic nerve and, analogously, increases TRPC5 expression and decreases axonal extension, suggesting that TRPC5 modulates the projection of cell axonal processes of ganglion cells during and after retinal development (Oda et al., 2020).
TRPC1, C4, and C7 are expressed in the RPE, as represented in Figure 2D. Intracellular Ca2+ currents decreased considerably with specific TRPC subfamily blockers, suggesting that the basal Ca2+ influx, essential for RPE functions, is mainly due to the activity of TRPC (Wimmers and Strauss, 2007; Cordeiro and Strauss, 2011).
TRPM1 has been described in the INL, specifically in ON bipolar cells, as well as in the OPL of mouse, macaque, and human retina (Choi et al., 2015; Klooster et al., 2011; Križaj et al., 2010; Morgans et al., 2009; Figure 2D). It is well documented that ON bipolar cells hyperpolarize in response to glutamate, released in the dark, due the activation of mGluR6. The molecular mechanism involves the closure of TRPM1 through alpha and the beta-gamma complex of the Go-protein stimulated by mGluR6 (Koike et al., 2010; Xu et al., 2016). Thus, ON-bipolar cells show a depolarizing response to light, with the decrease of glutamate release. In agreement, TRPM1 knockout in mice results in a clinical pattern similar to the night blindness pathology (Morgans et al., 2009; Irie and Furukawa, 2014).
Previous studies have described how Mg2+ modulates some members of the TRP family (Huang et al., 2020; Sawamura et al., 2017; Zierler et al., 2017). Intracellular Mg2+ (1.7–2 mM) decreases the TRPM1 current in rod and ON-cone bipolar cells, and stimulation of the PLC-PKCα pathway alleviates this inhibition only in rod bipolar cells (Rampino and Nawy, 2011).
Recently, studies addressed the effects of TRPM2-mediated calcium influx when the channel was stimulated by oxidative stress, as well as the neuroprotective effect of antioxidants for TRP modulation in ARPE-19 cells and a role on sirtuin 2-dependent cell death (Figure 2D; Meléndez García et al., 2015; Daldal and Nazıroğlu, 2022a, 2022b).
In mice, TRPM3 has been found in the iris, ciliary body, retinal pigment epithelium, GCL, INL, IPL, and OPL (Brown et al., 2015; Hughes et al., 2012; Webster et al., 2020; Figure 2D). Expression of TRPM3 has also been described in Müller glial cells during mouse retinal development (Webster et al., 2020). A recent study showed an increase in Ca2+ influx induced by TRPM3 agonist into TRPM3-positive ganglion cells in developing mice, whereas TRPM3 knockouts presented a reduction in these currents (Webster et al., 2020). Knockouts for TRPM3, but not for TRPM1, have shown a lower percentage of pupil contraction in response to low light intensity stimuli when compared to control mice (Hughes et al., 2012).
TRPM4 is detected in the Müller glial, GCL, ONL, and, specifically, in cone photoreceptors of the human retina (Berdugo et al., 2021; Figure 2D). In addition, TRPM4 in the outer limiting membrane of the human retina partially colocalizes with internal rectifier potassium channel 6.2 (Kir6.2) and sulfonylurea receptor (SUR1), both subunits being constituents of ATP-dependent potassium channels (Berdugo et al., 2021; Omri et al., 2010). SUR1 or Kir6.2 also co-localizes with Müller glial cells and cone photoreceptors in monkey retinas (Berdugo et al., 2021).
RPE undergoes blue wavelength-induced apoptotic cell death. Blue light is emitted by sunlight and light-emitting diode (LED) lamps (Hu and Xu, 2021; Tao et al., 2019). TRPM7 overexpression protects RPE cells from cell death induced by blue light, while TRPM7 ablation results in a low proliferation rate and lower cell viability, a decrease in the levels of PKC, ERK, and Bcl-2 proteins, and an increase in Bax (Hu and Xu, 2021). These data suggest a key role for TRPM7 in the survival of RPE cells exposed to blue light via PKC/ERK signaling (Hu and Xu, 2021).
TRPP2 has been detected in all layers of the mouse retina, with strong intensity in the outer segments of cones and INL (Gilliam and Wensel, 2011; Figure 2E). Patients with ML-IV have severe deleterious neurological, motor, and ocular consequences (Smith et al., 2002; Grishchuk et al., 2016). In the genetic ablation of TRPML1, it was seen that there was a thinning of the ONL and shortening of the outer segments of the rods in an age-dependent manner. Furthermore, except for INL, accumulation of lysosomal aggregates was found in almost all retina layers in Trpml1−/− (Grishchuk et al., 2016).
In animals with retinal detachment, agonist stimulation of TRPML1 decreases rod and cone apoptosis, preserving the photoreceptor layer. In addition, the activity of the channel changes in these retinas and therefore, ROS concentrations are markedly smaller. In this way, there is an improvement in the visual capacity in the injured animals treated with an agonist. These results make the channel an excellent therapeutic target (Yan et al., 2021).
Except for TRPML, TRPP1-3 mRNA expression has been demonstrated in the optic nerve. Furthermore, TRPP1-2 is one of the most abundant in astrocytes around the ocular structure (Choi et al., 2015). Mucolipidosis IV can also induce optic nerve thinning and neuronal demyelination at the level of the brain, which results in severe motor problems (Smith et al., 2002; Grishchuk et al., 2016). As expected, TRPML1 knockout mice show a decrease in the thickness of myelinated axonal projections and optic nerve thickness, possibly caused by a decrease in blood supply (Grishchuk et al., 2016).
Toll-like receptor 3 (TLR3) has recently been linked to ATP production from lysosomes in RPE cells and astrocytes expressed in the murine optic nerve head (ONH). The activation of TLR3 in these cells induces the stimulation of TRPML1, which, in turn, by inducing the release of lysosomal Ca2+ into the extracellular environment, can promote the fusion of the plasma membrane with the lysosomal membrane and increase the release of ATP from the organelle (Beckel et al., 2018). Gomez and colleagues (2018) observed, in Trpml1−/− mice, low extracellular Ca2+ concentrations even with pharmacological stimulation and confirmed that ion efflux occurred through channel activity (Gómez et al., 2018).
It is known that the inadequate functioning of the lysosome can lead to the accumulation of metabolites, including lysosomal lipids, which generate oxidative stress in the tissue. Experimental data indicate that TRPML1 gene expression remained at normal levels in RPE, but extracellular Ca2+ elevation was impaired under conditions of lipid accumulation, suggesting that TRPML1 responses are essential for lysosomal ATP release (Gómez et al., 2018).
Finally, TRPVs are expressed in astrocytes, microglia, and endothelial cells (Ho et al., 2014; Leonelli et al., 2010). TRPV1 is located in all retina layers during development and in adult mice. V1 shows higher immunofluorescence intensity in INL, GCL, and IPL in the mature mouse retina, while V2 is weakly restricted to INL, OPL, IPL, and GCL (Choi et al., 2015; Leonelli et al., 2010; Leonelli et al., 2009). As illustrated in Figure 2C, TRPV1 is expressed in amacrine cells, and TRPV4 was recently found in bipolar cells, while the V4 homodimer was found in Müller glia and RCGs. However, there are also reports of TRPV1/TRPV4 co-localization in these regions of the retina of mice (Gao et al., 2019; Leonelli et al., 2011; Sappington et al., 2015).
In the retina, TRPVs play a role in mechanical damage (Ho et al., 2014; Leonelli et al., 2010). As reported by Ho et al. (2014), in astrocytes that express TRPV1 and when scratching retinal tissue, there is an increase in the shortening and migration of these cells, through an increase of intracellular Ca2+ mediated by the channel in response to mechanical stress (Ho et al., 2014).
Interestingly, TRPV1 levels increase in the GCL and optic nerve axons after optic nerve injury in mice, whereas 21 days after injury, there is a decrease in TRPV1 staining in microglia and astrocytes. On the other hand, capsaicin, a TRPV1 agonist, accelerated cellular degeneration, induced retinal thinning, and intensified glial fibrillary acidic protein (GFAP) immunofluorescence in Müller’s glia. Capsaicin increases the generation of lipid peroxidation products in vitro bu
Comments (0)