Berylliosis is an acquired type IV hypersensitivity reaction to beryllium exposure, generally in an occupational setting.
Berylliosis presents with nonspecific respiratory symptoms, including shortness of breath and dry cough, that mimic other conditions, particularly sarcoidosis.
An occupational history should be obtained when investigating patients with respiratory symptoms.
A beryllium lymphocyte proliferation test is the only current diagnostic test capable of differentiating berylliosis from sarcoidosis.
A 56-year-old female presented to her family doctor with insidious shortness of breath that had progressed over several months, from exertional dyspnea to shortness of breath at rest. She had no fever, night sweats, weight loss nor hemoptysis, and was otherwise asymptomatic. At the time of presentation, her medical history included sleep apnea and a mood disorder, which was being treated with a serotonin–norepinephrine reuptake inhibitor. She had never smoked. She had been working as a welder for more than 3 decades in the oil and gas sector. She used various welding methods and metals, including some self-described “exotic metals,” in indoor and outdoor facilities with poor ventilation. She wore respirators infrequently. Her family doctor referred her to a respirologist at the regional hospital for further investigations (Table 1). Gaps in time between investigations occurred in part because the patient moved among provinces during management.
Table 1:Summary of investigations for a 56-year-old welder, with associated differential diagnosis
We ordered a pulmonary function test, which showed mild diffusion capacity impairment, commensurate with numerous possible diagnoses (Table 2). The patient’s complete blood count, coagulation, liver function, electrolytes, renal function and thyroid-stimulating hormone levels were normal. A chest radiograph showed nonspecific subsegmental atelectasis in the left lower lung zone. A computed tomography scan of the chest showed multiple 2- to 3-mm noncalcified pulmonary nodules, notably one 18-mm, ill-defined, ground-glass nodule in the left lower lobe and one 7-mm nodule in the right upper lobe. Hilar and mediastinal lymph nodes were slightly prominent but not enlarged beyond normal limits. We saw no reticular markings, abnormal septal thickening, emphysema, volume loss or pleural effusions. The imaging results suggested a previous granulomatous infection, interstitial lung disease or malignancy.
Table 2:Pulmonary function test results
Over the next 6 months, we collected 3 sets of transbronchial biopsies of lung and lymph node tissue, using bronchoscopy without fluoroscopy. The first biopsies showed normal tissue with no evidence of infection. Because inflammatory diseases are known for heterogeneity in granuloma formation, repeat biopsy sampling is often pursued when a reasonable suspicion persists. The second biopsies were of suboptimal quality. Therefore, we obtained a third set, which showed circumscribed, non-caseating granulomas within the patient’s lung tissue and lymph nodes (Figure 1). The third biopsies were negative for acid-fast bacilli (Ziehl–Neelsen), fungi (Grocott’s methenamine silver stain) and polarizable foreign material. Additional investigations for infection, including bronchoalveolar lavage, of routine bacterial and mycobacterial stain and culture were negative. Our differential diagnosis narrowed to inflammatory interstitial lung disease.
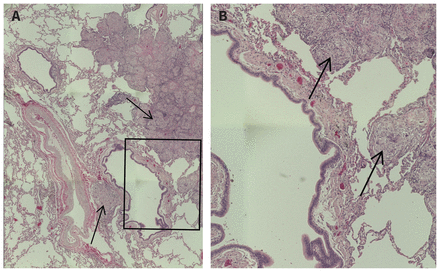
 Figure 1:
Figure 1: Circumscribed, non-necrotizing granulomas from left lower lobe lung tissue of a 56-year-old female. (A) Low-power magnification of lung tissue with granulomas (arrows) around bronchovascular bundles and interlobular septae (original magnification × 100); (B) high-power magnification of specific lung tissue region (boxed region from (A)) with granulomas (arrows) (original magnification × 200).
To distinguish between the main 2 suspected causes of interstitial lung disease, sarcoidosis and berylliosis, we performed a bronchoalveolar lavage beryllium lymphocyte proliferation test (BeLPT). The results showed a dose–response proliferation of lymphocytes to beryllium sulfate, indicating a diagnosis of berylliosis (Table 3). The patient retrained in a different career (as a truck driver), which offered the simplest solution to confidently minimize beryllium exposure. Given her minimal pulmonary impairment at time of diagnosis, aided by time away from welding work, we prescribed no pharmacologic treatment.
Table 3:Laboratory results from beryllium lymphocyte proliferation test on bronchoalveolar lavage
We monitored the patient’s lung function over the subsequent year with 3 pulmonary function tests, which showed reduced lung diffusion capacity that worsened and then improved (Table 1). The patient experienced an episode of migratory chest discomfort and breathlessness 9 months after diagnosis, which resolved spontaneously; a ventilation perfusion scan showed no pulmonary emboli. We recommended regular follow-up with her family doctor for re-emergence of respiratory symptoms, and annual pulmonary function tests until the results are clearly stable.
DiscussionBeryllium is a low-molecular-weight metal that is widely used by itself and as an alloy because of its favourable properties for welding. 1 Most exposure occurs in the workplace; high-risk industries include construction (welding, abrasive blasting), automotive, ceramics, computer circuit board recycling, electronics, the oil and gas sector, industrial machinery repair, jewellery-making, metal fabrication and mining.1
Occupational exposureOccupational exposure to beryllium occurs primarily through inhalation of airborne particles.1 At present, there is no safe level of exposure to beryllium, which is designated as carcinogenic to humans (Group 1) by the International Agency for Research on Cancer.2 The current American Conference of Governmental Industrial Hygienists’ threshold limit value for beryllium is 0.05 μg/m3 in workplace air, reflecting the proposed average airborne concentration a healthy person can inhale per shift over the course of their career without adverse health effects.2 Welding is known to be high risk for beryllium exposure: 1 study that included various industries found welders had a mean beryllium 8-hour time-weighted average exposure equivalent to 0.24 μg/m3 over their full shift.3 If using a half-face respirator with P100 particulate filters (which filter out 99.97% of all particles), the exposure would be reduced to 0.024 μg/m3, below most Canadian occupational exposure limits (0.05 μg/m3). Employers are required by law to ensure occupational hazards are adequately controlled by implementing preventive measures that follow a specific sequence (Table 4).
Table 4:Preventive measures to minimize occupational exposure to beryllium
Consequences and clinical features of exposureBeryllium exposure primarily causes beryllium sensitization and berylliosis (also called chronic beryllium disease), both of which are an acquired type IV hypersensitivity reaction to beryllium. Beryllium sensitization is similar to berylliosis but without lung pathology. Berylliosis causes granulomatous interstitial lung disease, with transient inflammation and granulomas. 4 The clinical features of both are mainly nonspecific shortness of breath and dry cough; fever, night sweats, clubbing and weight loss are less common.4 Symptoms generally improve when exposure stops. The HLA-DPB1 gene polymorphism with glutamic acid at the 69th codon is associated with an increased risk of developing berylliosis by up to eightfold in patients with a history of exposure to beryllium; it has a general population prevalence between 15% and 25%.4,5 In practice, genetic screening in high-risk occupational groups is not routinely done, owing to ethical issues regarding employment and insurance discrimination.
Prevalence, risk factors and prognosisReporting of berylliosis is limited, dated and thought to be underdocumented.6 The prevalence of berylliosis in beryllium-exposed populations is estimated to be between 2% and 5%, but can be as high as 15% of workers in high-exposure occupations, such as machinists.7 Given that berylliosis mimics sarcoidosis clinically, pathologically and radiologically, it is estimated that about 6% of patients diagnosed with sarcoidosis may instead have berylliosis.6 This likelihood increases to 40% when beryllium exposure is established.6 Sarcoidosis prevalence in 2015 in Ontario was 143 per 100 000, which suggests that berylliosis prevalence could be as high as 8 per 100 000 in the general population, and higher in specific worker populations.8
The major risk factor for berylliosis is beryllium exposure. Sensitization and symptom onset may be delayed after exposure, from 3 weeks to 30 years.1 Berylliosis severity and prognosis are variable, but are worse with higher exposure intensity, metals that contain a higher percentage of beryllium, and more lymphocytes in bronchoalveolar lavage samples.9 If symptoms improve after exposure avoidance or pharmacologic treatment, these improvements generally remain as long as avoidance or treatment continue. Despite treatment, some patients progress to pulmonary fibrosis. In 1 cohort study, 8 patients with berylliosis had evidence of pulmonary fibrosis before treatment; the fibrosis of 6 patients did not progress on corticosteroid treatment.4
InvestigationsNonspecific investigations for berylliosis include pulmonary function tests and lung imaging, which may show lung diffusion capacity limitation and perilymphatic lymphadenopathy with or without ground-glass pulmonary nodules, respectively. Initial investigation for beryllium sensitivity can be done by 2 peripheral blood BeLPTs (68.3% sensitivity, 96.9% specificity); 2 samples are taken simultaneously, owing to low test sensitivity and diagnostic criteria.1,10 If both results are negative, surveillance of respiratory symptoms, pulmonary function tests and beryllium sensitization (via serum BeLPT) can be done for high-risk patients.1 If negative or borderline results are obtained and berylliosis is still suspected, a bronchoalveolar lavage BeLPT will often confirm diagnosis.1 A bronchoalveolar lavage BeLPT may be favoured as the initial investigation when there is evidence of abnormal pulmonary function tests or imaging studies.1
To diagnose berylliosis, there must be a history of beryllium exposure, histopathologic findings consistent with berylliosis (non-caseating granulomas) and a positive BeLPT (serum or bronchoalveolar lavage).1 Lung biopsies have a 5%–10% false-negative rate and may require resampling.11 The BeLPT can distinguish sarcoidosis from berylliosis, as most other findings will be similar. The BeLPT measures lymphocyte cell count in serum or bronchoalveolar lavage samples after exposure to beryllium. A positive result on this test indicates exaggerated lymphocyte proliferation in response to beryllium. National Jewish Health in Colorado performs most bronchoalveolar lavage BeLPT tests in North America, with test sensitivity at 97.2% or greater.12
Management and secondary preventionOral glucocorticoids are the first-line pharmacologic treatment for berylliosis, starting with 20–40 mg daily doses for 3 months, then tapering down 5 mg per week to the lowest effective dose.11 Glucocorticoid regimens are based on a limited number of case reports and series.4 Methotrexate is used for patients who are glucocorticoid intolerant or refractory, starting with 7.5 mg weekly, then increasing by 2.5 mg biweekly until 15 mg weekly.11 The use of methotrexate is based on its use in sarcoidosis. 11 Managing berylliosis should prioritize eliminating workplace exposures through reassignments (Table 4). In practice, exposures are mostly controlled with respirators. Generally, half-face respirators with P100 filters are sufficient if well fitted and used consistently and properly.
Clinicians should consider an occupational cause for respiratory symptoms whenever shortness of breath or cough are present. Collecting information thorough occupational histories includes exploring symptom–workplace associations, potential for exposures, workplace tasks and preventive measures (Table 5). This is particularly important for patients suspected of having, or who have received a diagnosis of, sarcoidosis.
Table 5:Essential information for occupational respiratory history
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
FootnotesCompeting interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: All of the authors contributed to the conception and design of the work. Matthew Loss drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
Comments (0)