A 25-year-old man presented with complaints of giddiness and severe headache for 20 days. Magnetic resonance imaging showed a solid mass lesion of size 56 × 57 mm in the left frontal lobe. Intraoperative tumor cavity fluid (2 mL, clear) was aspirated and sent for cytological examination. Cytocentrifuge smears prepared were cellular [Figure 1a-c].
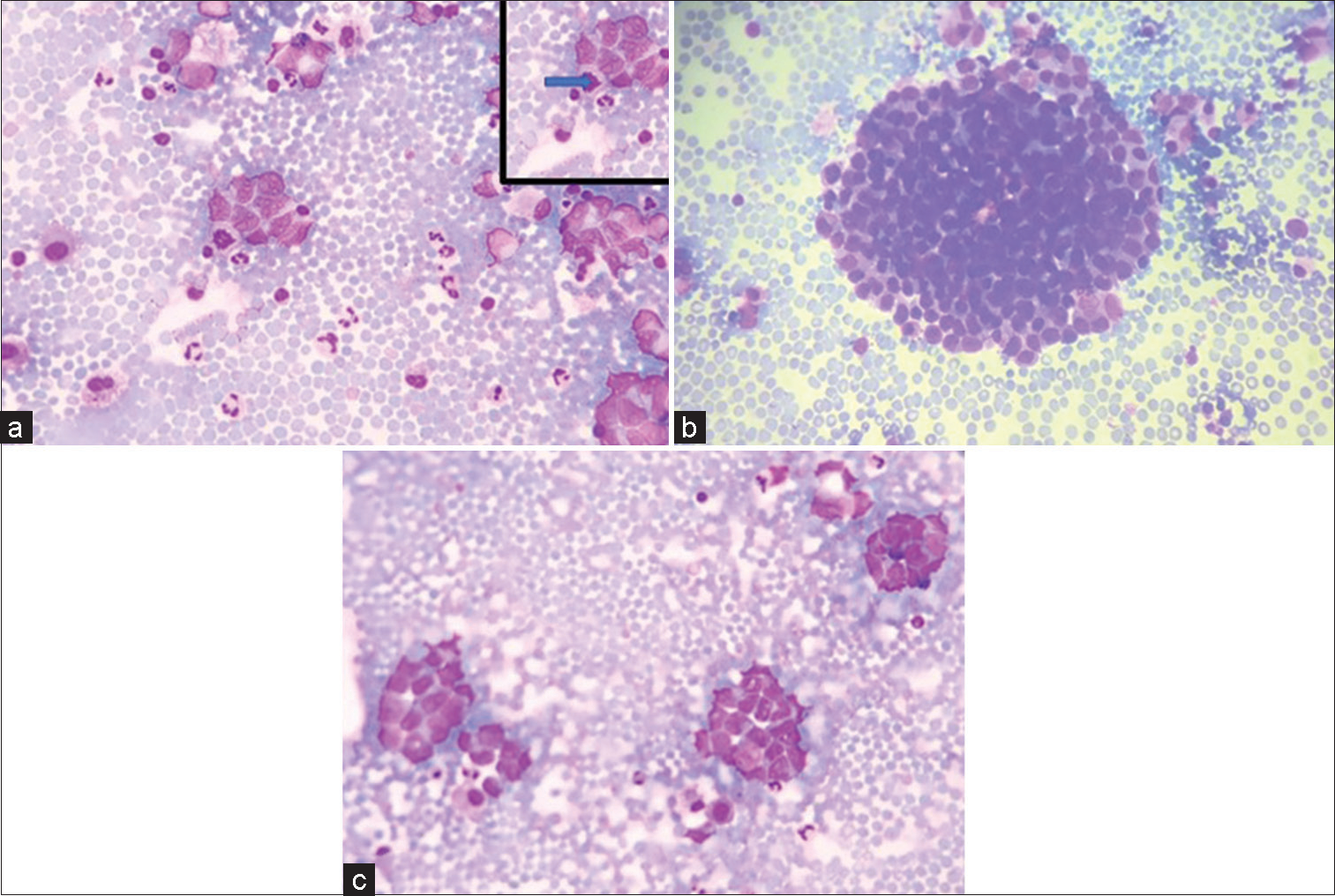
Figure 1:: (a-c) (GIEMSA ×40) Smears showing tumor cells in clusters and singly scattered having high N:C ratio and opened up chromatin and pleomorphism. (a) Inset shows atypical mitoses pointed by solid arrow.
Export to PPT
QUESTION 1 What is your interpretation?Degenerated cells
Adenocarcinoma
Glioma
Small blue round cell tumor.
ANSWER TO QUESTION 1The correct cytopathological interpretation is d. Small blue round cell tumor.
EXPLANATIONCytocentrifuge smears prepared were cellular comprising of monomorphic tumor cells arranged in clusters and nests. Individual tumor cells are round to ovoid having scant to moderate amount of delicate cytoplasm. Nuclei were regular, smooth with opened up chromatin, showing mild-to-moderate degree of pleomorphism [Figure 1a-c]. Atypical mitosis was noted. However, rosette or acini formation or necrosis or moulding was not seen.
The cells were cohesive with no acini formation, delicate chromatin and no nucleoli thereby ruling out adenocarcinoma (Option b).
No glial matrix or fibrillary processes or rosenthal fibres or vessels or calcification or cellular pleomorphism or endothelial cell proliferation were displayed hence possibility of glioma was also excluded from the study (Option c).
Degenerated cells (Option a) were seen but most of the cells were preserved and had distinct cell boundary with nuclear margins and cellular details as discussed briefly above. Based on findings described above, a provisional diagnosis of small blue round blue cell tumor was considered. Cell block and immunocytochemistry could not be performed due to exhaustion of fluid while making smears for routine examination.
Excision surgery was performed and histopathological examination revealed a tumor [Figure 2a and b].
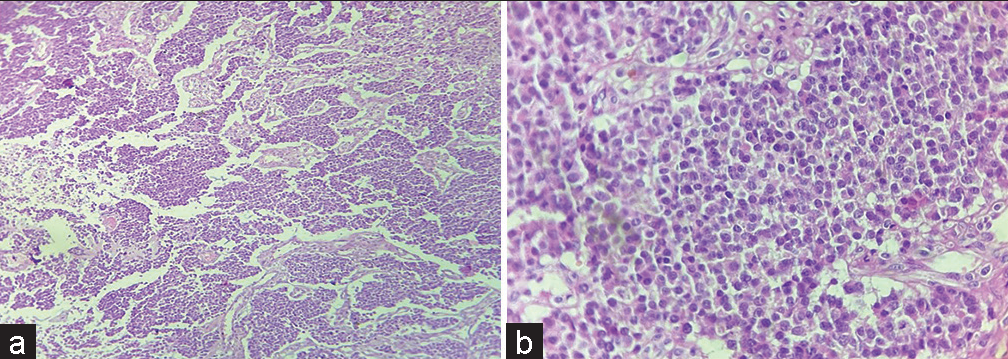
Figure 2:: (a) Tumor cells arranged in nest, chords and trabeculae (H & E ×10); (b) Tumor cells having round nucleus and powdery chromatin (H & E ×40).
Export to PPT
QUESTION 2All of the following are among the differentials except
Germ cell tumor
Astrocytoma
Melanoma
Neuroendocrine tumor.
EXPLANATIONHistopathological examination revealed tumor cells arranged in nests, chords and trabeculae showing moderate degree of pleomorphism. Cells were round to oval with stippled nuclear chromatin [Figure 2a and b]. Areas of hemorrhage and focal areas of necrosis were also seen. Mitotic figures of 2–3/10 high power field noted. The various differential included are neuroendocrine neoplasm (NEN), germ cell tumor, melanoma, and lymphoma.
Astrocytoma (option b) will show tumor cells having irregular nuclei with variable degree of atypia along with presence of glial processes. Cells can have variable cellular morphology in a fibrillary background which is not seen in this case.
Initial panel of immunohistochemistry (IHC) was applied which included Glial fibrillary acidic protein (GFAP), LCA, SALL4, CK7, CK20, CD117, desmin, myogenin, CD99, HMB 45, and S-100. All above markers came out to be negative except focal pan-cytokeratin positivity [Figures 3a-e]. A secondary panel of synaptophysin and chromogranin applied was positive. KI67 proliferation index was >20%.
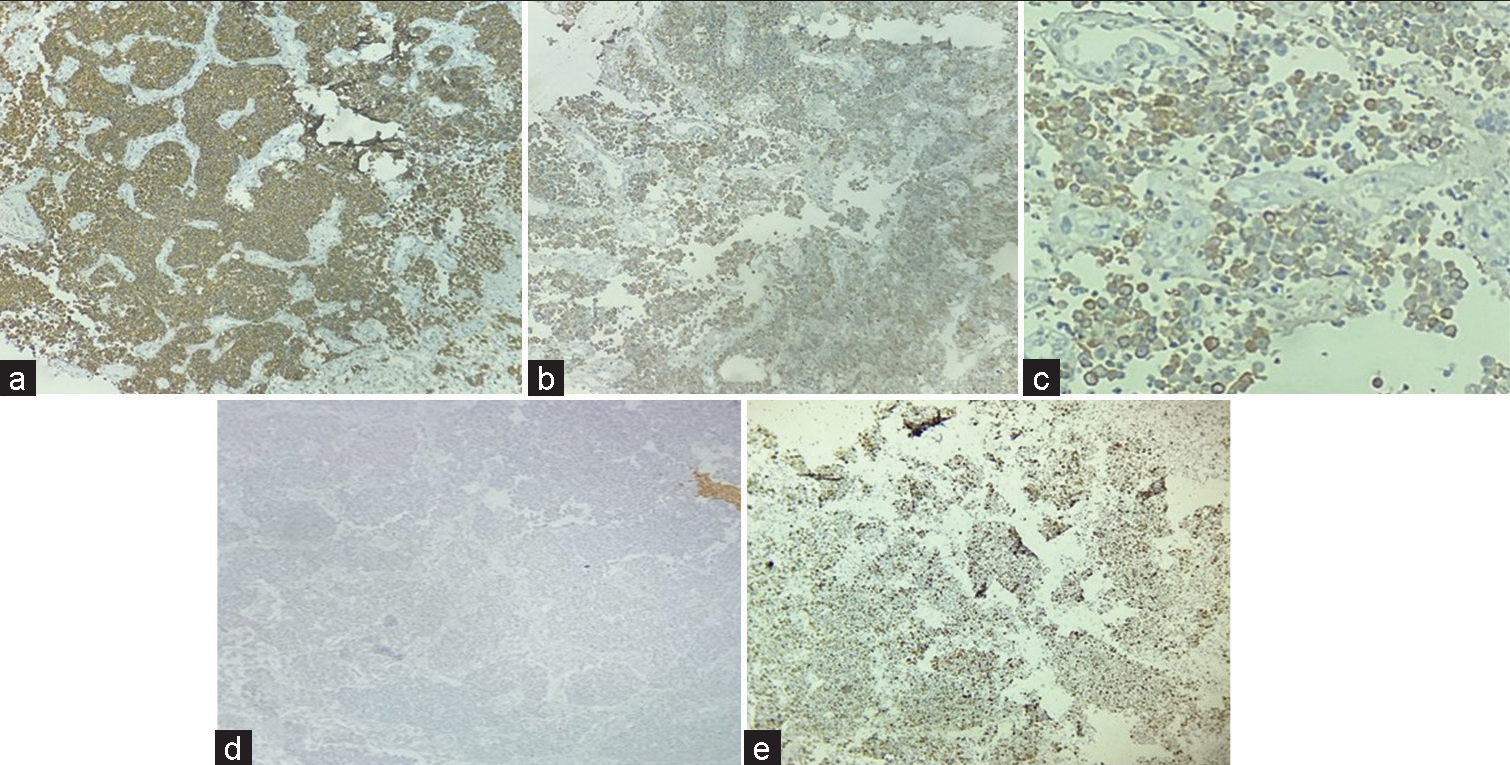
Figure 3:: Immunohistochemistry: Strong positive synaptophysin (a), Positive Chromogranin (b), Pancytokeratin focal positive (c), and Glial fibrillary acidic protein negative (d), Ki67 >20% (e) (×40).
Export to PPT
QUESTION 3Which marker helped in excluding the primary nature of the lesion?
Synaptophysin
Chromogranin
Pan-cytokeratin
GFAP.
EXPLANATIONGFAP is positive in intermediate filament of astrocytes. In the present case, it was negative in the tumor cells.
Synaptophysin and chromogranin are markers for neuroendocrine cells whereas pancytokeratin indicates the epithelial nature of the lesion.
QUESTION 4All further IHCs can be put to check for the metastatic site of NEN except
Insulinoma associated protein 1 (INSM-1)
CDX2- Caudal type homeobox2 (CDX2)
Thyroid transcription factor 1 (TTF-1)
Pancreatic battery (Insulin, gastrin, somatostatin, glucagon, etc.).
EXPLANATIONInsulinoma associated protein 1 (INSM-1) is a nuclear marker of neuroendocrine differentiation with better sensitivity and specificity as compared to synaptophysin, chromogranin, and CD56.
CDX2 helps in location metastasis from colon whereas TTF-1 from lung.
On extended IHC, the cells were focally positive for TTF-1 [Figure 4] while negative for Napsin-A and CDX2.
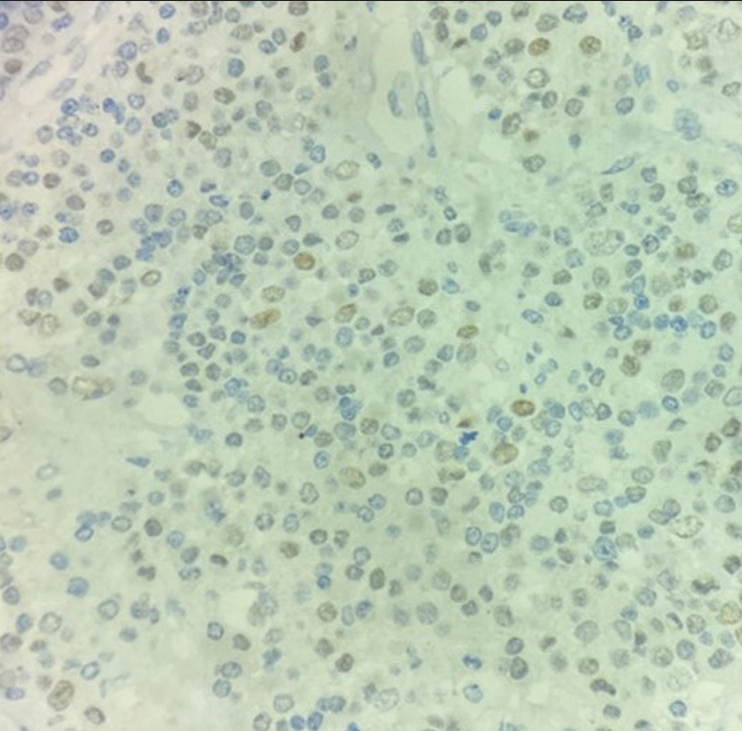
Figure 4:: Tumor cells are positive for TTF-1 (×40).
Export to PPT
FURTHER FOLLOW UP OF THE CASEA Positron emission tomography/Computed tomography (PET/CT) was performed which showed metabolically active soft tissue density lesion measuring 2.5 × 1.7 × 2.5 cm in the upper lobe of left lung in the para mediastinal aspect with metabolic activity in the mediastinal lymph nodes. The above PET/CT findings further confirmed the histopathological diagnosis of tumor arising in lung.
BRIEF REVIEWNEN can develop at any of the initial mentioned systems where the neuroendocrine cells are present.[1] Incidence of central nervous system (CNS) metastasis is very rare and accounts 1.5–5% of all patients[2] where NEN source found to be in 1.3–1.4% in all cases of CNS metastasis.[3] It is always a challenging task to detect primary focus, because patients have specific symptoms when the tumor size is small. Radiological investigations including somatostatin scintigraphy and PET/CT proved to be very useful. Metastatic disease in addition to differentiation and proliferation rates is an important prognostic factor in NEN. Presence of brain metastasis is usually found to be characteristic of a systemic dissemination and disease progression.
Cytological features of neuroendocrine tumors are described in literature that includes monomorphic population of cells, loosely cohesive fragments, medium in size having abundant cytoplasm, and very few mitotic figures. Occasional rosette formation can be seen. Nuclei were regular, smooth with opened up chromatin, showing mild-to-moderate degree of pleomorphism [Figure 1]. Occasional cells showed prominent nucleolus. Atypical mitosis was seen. Due to variation in the cytomorphological features of neuroendocrine tumor, they mimic a variety of tumors. The features range from discrete cells to tight cohesive clusters, hyperchromatic nuclei with basophilic to granular to scant cytoplasm. Nuclear moulding may or may not be seen. Pleomorphism can be seen.[4]
In the present case, diagnosis of metastatic neuroendocrine tumor Grade 3 was made. TTF-1 positivity pointed out the brain tumor was metastatic, which was later confirmed by PETCT that exhibited a primary lesion in the lung. The grading of neuroendocrine tumors (NET) is based on Ki67 proliferation index. However, in neuroendocrine carcinomas, also it is advised for distinguishing it from Grade 3 NETs.[5] Features favoring neuroendocrine carcinoma are dirty smear with areas of necrotic debris and very scant cytoplasm in small cell type whereas abundant in large cell type. The population of cells having salt and paper chromatin, severe nuclear fragility, and many atypical mitoses.[2,6] Only limited previous case reports are available discussing NEN initial presentation as brain metastasis,[7-11] one of which was reported as a primary brain NEN of third ventricle adjacent to which in the paraventricular nucleus resides group of neuroendocrine cells.[12] Mostly by the time patient presents with brain metastasis either metastasis to lung happens or develop primary lung tumor. The metastasis to brain occurs due to hematogenous spread of the tumor. Leading cause of death in patients with CNS NENs is secondary to systemic disease progression and a better 10-year overall survival rate is seen in primary brain NENs.[13] Imaging workup holds importance where CT imaging has sensitivity of 95% in identification of primary tumor.[14] Etoposide -platinum chemotherapy is the treatment of choice in grade 3 NEN.[15] In metastatic brain NENs, radiation therapy and surgery are found to be beneficial but more research is required for proper management of primary brain NENs.[16]
SUMMARYMetastatic disease is a major prognostic factor in NEN s in addition to differentiation and proliferation rate. Brain metastasis of neuroendocrine carcinoma is a rare entity but the possibility of it should be borne in mind for accurate diagnosis. In the present study, patient has been on regular follow-up for 9 months and is living disease free while continuing on etoposide chemotherapy.
留言 (0)