Acute pulmonary embolism (PE) affects 0.1% of the U.S. population annually and is associated with significant morbidity and mortality nationwide.[1] PE arises from venous thrombi, most commonly found in the lower extremities, which dislodge and cause occlusion of the pulmonary arteries. The risk factors of PE are genetic, prolonged immobilization, and hypercoagulable states. The most common PE symptoms at presentation are acute chest pain and dyspnea.[1] The reasoning behind such presentation is because patients with PE have a ventilation perfusion (V/Q) mismatch resulting in shortness of breath. Chest pain and cough are often associated with production of bloody sputum due to the clot compromising the lungs’ blood supply.
In hemodynamically unstable patients, reperfusion strategies such as chemical thrombolysis and/or mechanical thrombectomy are required. Anatomically, PE can be classified as saddle, lobar, and distal. Saddle embolus refers to a large clot in the main pulmonary artery. Lobar involves a clot in either right or left pulmonary artery and distal PE affects the smaller branches: segmental and subsegmental. The decision to pursue treatment is determined by the clinician’s expertise.
In the emergency department (ED), symptoms of chest pain and dyspnea are non-specific and thus pose a challenge in making a definitive diagnosis. Therefore, radiological imaging is almost often used in conjunction with clinical decision rubrics such as Wells criteria, the PE rule-out criteria, the revised Geneva score, the simplified revised Geneva score, and a D-dimer test.[2] The current gold standard for imaging is contrast-enhanced computed tomography angiography (CTA), as it is widely available and has short scanning times in the ED setting, playing a crucial role in triaging severely dyspneic patients.[3] According to the American College of Radiology (ACR) Appropriateness Criteria, contrast-enhanced CTA of the chest is preferred over non-contrast CTAs. Non-contrast CTAs are limited to recognizing centrally located emboli and lack the ability to assist the clinician in appropriately identifying segmental and subsegmental clots.[4-6] Non-contrast CT of the chest effectively evaluates lung parenchyma, pleura and chest wall, but without contrast, there may be an inability to correctly discern calcified lesions from clots.
In late spring of 2022, a global shortage of iodinated contrast media occurred due to a COVID-19-induced global supply chain disruption. This iohexol (Omnipaque©, General Electric) shortage affected many clinical institutions that relied on this contrast agent for CT examinations, across the months of May to June. Due to the iohexol contrast shortage, our institution had to modify existing magnetic resonance imaging (MRI) MR angiography (MRA) chest protocols to meet the diagnostic quality standards in the ED patients presenting with characteristic symptoms. As a result, MRA was used as an alternative diagnostic tool over CTA in patients suspected of PE. The revised 2022 ACR Appropriateness Criteria suggests the use of contrast-enhanced MRA as an appropriate secondary diagnostic study for PE due to the iodinated contrast shortage.[7]
Figure 1 depicts an MRA image of the pulmonary vasculature. MRA is a safe, technically robust, and clinically effective test for the primary detection of PE. Recent advances in contrast-enhanced MRA techniques have led to increased use of this modality for the detection of PE in clinical setting.[8] MRA provides us with the ability to view alternative pathologies and incidental findings in various patients.[9]
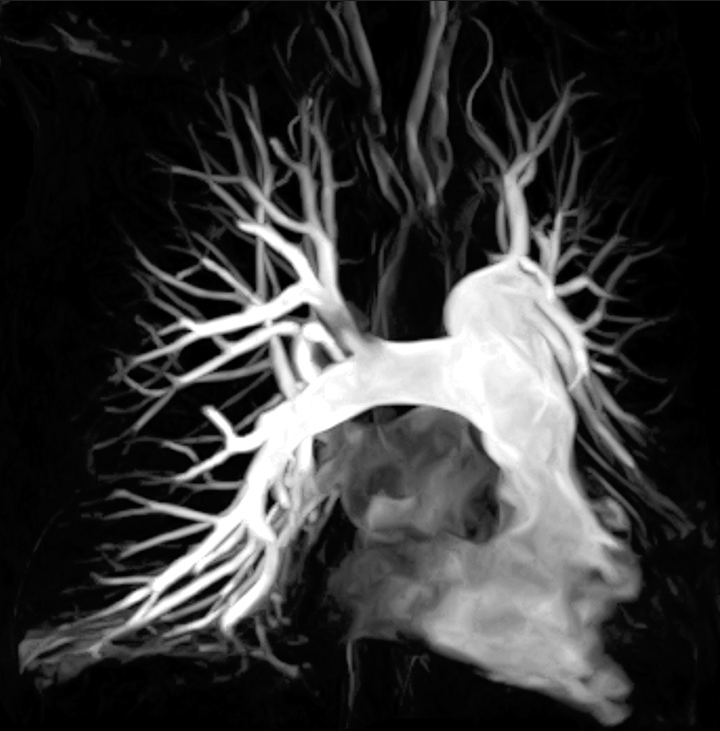
Figure 1:: A 53-year-old woman who presented with dyspnea. (a) Coronal post-contrast magnetic resonance angiography multiple intensity projection depicting good opacification of the pulmonary arterial branches to the subsegmental level.
Export to PPT
In this quality assurance (QA) study, we had three principal aims. The first was to compare the diagnostic potential between our alternative MRA protocols over the gold standard CTA. The second was to evaluate overall MRA imaging quality, and whether scanner type/imaging sequence made a difference in quality, in the setting of PE imaging. Finally, the third was to assess for any possible misdiagnosis risk in patients arriving to the ED with suspicion of PE during the 2 months of iodinated CT contrast shortage at our institute, the University of Florida (UF) College of Medicine, Jacksonville.
MATERIAL AND METHODS Data collectionWe obtained a certificate of registration from the Quality Improvement Project Registry per UF College of Medicine, Jacksonville guidelines for quality projects. We utilized mPower, a database software, which searches UF Health Jacksonville’s radiology studies and reports, to obtain a list of 62 patients who underwent a contrast-enhanced MRA of the chest during the time span of May 1, and June 30, 2022 at two of our campuses – 8th street downtown, and North campus. The list was filtered to include ED patients who underwent contrast-enhanced MRA chest imaging, performed for suspicion of PE, after duplicate entries were removed. Key words comprised shortness of breath, dyspnea, PE, deep venous thrombosis, clots, and right heart strain.
In addition, the patients’ electronic medical records (EMRs) was searched using EPIC to obtain information from their respective admission, such as PE risk from Wells score, and discharge diagnostic information, including radiographic images. We recorded initial complaint, as well as order indication, imaging sequence (time-resolved MRA or bolus chase MRA) depending on the scanner model used and lastly, contrast-enhanced MRA chest report results.
ImagingMRA acquisition was performed at our two ED campuses. Our downtown campus houses a Siemens MAGNETOM Vida 3.0T MRI, which uses the time-resolved angiography with stochastic trajectories (TWIST) sequence. Our North campus houses a Hitachi 1.2T Open MRI that uses the bolus-chase MRA sequence Fluoro-Triggered Examination. The imaging sequence parameters are shown in Table 1.
Table 1:: Sequence parameters.
Sequence parameters 1.2T Hitachi MR (FLUTE) 3.0T Siemens Vida MRI scanner (TWIST) Repetition time (TR) 4.4 ms 2.4 ms Echo time (TE) 2 ms 0.88 ms Slice thickness 4 mm 2 mm Number of slices 64 80 Matrix 256×256 352×246 Acquisition time 2 m, 43 s 3 m, 33 sWe removed seven results, as these did not indicate sufficient symptoms to have warranted to suspicion of PE, bringing the final sample size to 55. We collected the minimum amount of information necessary to answer our QA question. Data consisting of personal health information were excluded from the study. All data were secured and handled only by the research team.
Data classificationTwo fellowship-trained radiology faculties retrospectively reviewed the contrast-enhanced MRA chest images to verify the original report findings. As they reviewed images and reports, they also assessed the imaging quality using a modified version of the Prospective Investigation of PE III Likert scale; a six-point scale was adopted from Sostman et al., as shown in Table 2.[10] The radiologists reported the image quality based on the opacification of the main lobar, segmental and subsegmental pulmonary arteries as 1) good, 2) fair or 3) poor, per the guidelines, which assigned a 0 (best) to 6 (worst) ranking based on the sum total of scores. Moreover, the radiologists determined whether motion and/or suboptimal bolus were discernable in the images, as an additional measure of technical quality. The pretest probability for PE was classified as low risk (<2), medium risk (2–6), and high risk (>6), respectively, based off the Wells criteria.
Table 2:: Likert scale.
Main/lobar Segmental Subsegmental Score Good Good Good 0 Good Good Fair 1 Good Fair Fair 2 Good Good Poor 2 Fair Fair Fair 3 Good Fair Poor 3 Fair Fair Poor 4 Fair Poor Poor 5 Good Poor Poor 5 Poor Poor Poor 6 Poor Fair Poor 6 Statistical analysisWe employed Statistical Package for the Social Sciences (SPSS), version 26 (IBM® SPSS®) for all data analyses. We characterized the total sample based on the distribution of symptoms indicating suspicion of PE and categorized them. We utilized Chi-squared tests to evaluate individual associations between initial complaint (PE symptoms) and four dependent variables: (1) MRI sequence utilized, (2) risk score, (3) imaging quality issues noted, (4) vessel opacification Likert scale Score, and (5) imaging result. In addition, we also employed chi-squared tests to evaluate individual associations between imaging result and three dependent variables: (1) MR sequence, (2) quality issues, and (3) Likert scale score. We employed Cramer’s V to test for effect size in the comparisons. Finally, we used one-sample Wilcoxon signed rank test to locate the median of Likert scores to measure how far the quality scores are from 0 (best), with alpha set at 0.05.
RESULTSAcross the four initial complaints for suspicion of PE for which patient received an MRA of the chest, 89% were either categorized as presenting with chest pain or shortness of breath [Table 3]. Chi-squared tests did not find significant associations between initial complaint and the MR sequence chosen for the scan as well as the Wells criteria based risk score. In regard to scan quality issues, motion was noted in 80% of the 55 studies that were reviewed. Nonetheless, we found no significant association between the initial complaint type and the type of quality issue noted on imaging. There were significant associations (P < 0.009) between Likert scale scores and initial complaint [Table 3].
Table 3:: Sample distribution of ED patients that received an MRA PE protocol during May and June 2022 at UF Health Jacksonville.
Initial complaint n(%) MR sequence* Risk score** Quality issues Likert scale score Imaging result Bolus chase (%) TWIST (%) Low Medium High None (%) Motion (%) Motion and suboptimal bolus (%) 0 (%) 1 (%) 2 (%) 3 (%) 4 (%) 5 (%) 6 (%) Positive (%) Negative (%) Shortness of breath 22 (40) 11 (50) 11 (50) 9 7 6 3 (13.6) 17 (77.3) 2 (9.1) 4 (18.2) 9 (40.9) 1 (4.5) 4 (18.2) 3 (13.6) 1 (4.5) 0 (0) 1 (0.5) 21 (95.5) Chest pain 27 (49) 18 (66.7) 9 (33.3) 11 10 6 7 (25.9) 19 (70.4) 1 (3.7) 4 (14.8) 12 (44.4) 5 (18.5) 5 (18.5) 0 (0) 1 (3.7) 0 (0) 1 (3.7) 26 (96.3) Cough 3 (5.5) 1 (33.3) 2 (66.7) 1 1 1 1 (33.3) 2 (66.7) 0 (0) 0 (0) 2 (66.7) 1 (33.3) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 3 (100) Dizziness/Vomiting/Status Epilepticus 3 (5.5) 3 (100) 0 (0) 2 0 1 0 (0) 3 (100) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 1 (33.3) 1 (33.3) 1 (33.3) 0 (0) 3 (100) Total 55 (100) 33 (60) 22 (40) 23 18 14 11 (20) 41 (74.5) 3 (5.5) 8 23 7 9 4 3 1 2 (3.6) 53 (96.4) Cross tabulation Chi-Square P= 0.230 0.926 0.787 0.009 0.964 Cramer’s V= (df) 0.280 (3) 0.187 (6) 0.170 (6) 0.463 (18) 0.071 (3)Adjusted for degrees of freedom, the Cramer’s V results indicated a medium effect. We found no statistically significant associations between report findings and initial complaint category and report findings and the Wells criteria based risk score. Finally, when evaluating associations between imaging results and three dependent variables, we found no significant differences between positive or negative results in regard to what MR sequence was used for imaging, whether any quality issues were noted on the scan or not, and their vessel opacification Likert scale score [Table 4]. One-sample Wilcoxon test indicated significant difference for the median Likert scores for 0 and 1. However, the difference was not significant for the median score 2, which corresponds to “good-fair” in the Likert scale for the MRA quality.
Table 4:: Sample distribution of ED patients that received an MRA PE protocol during May and June 2022 at UF Health Jacksonville.
Imaging result MR sequence* Quality issues Likert scale score Cross tabulationThis QA study was done to ensure that patients scanned with MRA were not compromised in terms of diagnostic potential. The iodinated contrast shortage period provided us with an opportunity to explore alternative diagnostic imaging at a time of critical need to test protocols that would allow us to continue providing optimal patient care. MRA has been found to be as effective if not superior to CTA in terms of not requiring the use of ionizing radiation and MRA being associated with fewer adverse events within the first 6 months after the exam.[9] In addition, gadolinium dye used in MRA is less nephrotoxic and anaphylactic in comparison to the iodinated contrasted utilized in CT imaging.[11,12] In addition, Repplinger et al. assert that their institution offers a focused 10-min MRA examination as a clinical service for primary acute PE.[9] During our study, the incidental findings consisted of a mycotic aneurysm [Figure 2] and myofibroblastic tumor [Figure 3].
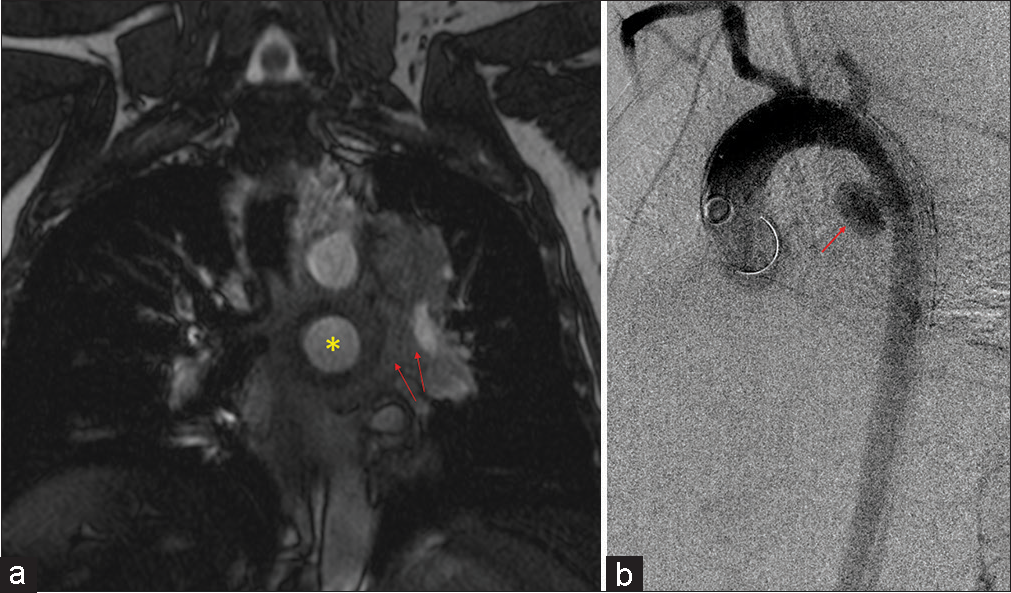
Figure 2:: A 47-year-old male who presented with chest pain. (a) Coronal post-contrast magnetic resonance angiography sequence showing a large saccular aneurysm arising from the proximal descending aorta (yellow star) with periaortic hematoma (red arrows). (b) Sagittal aortogram confirming large saccular mycotic aneurysm (red arrow).
Export to PPT
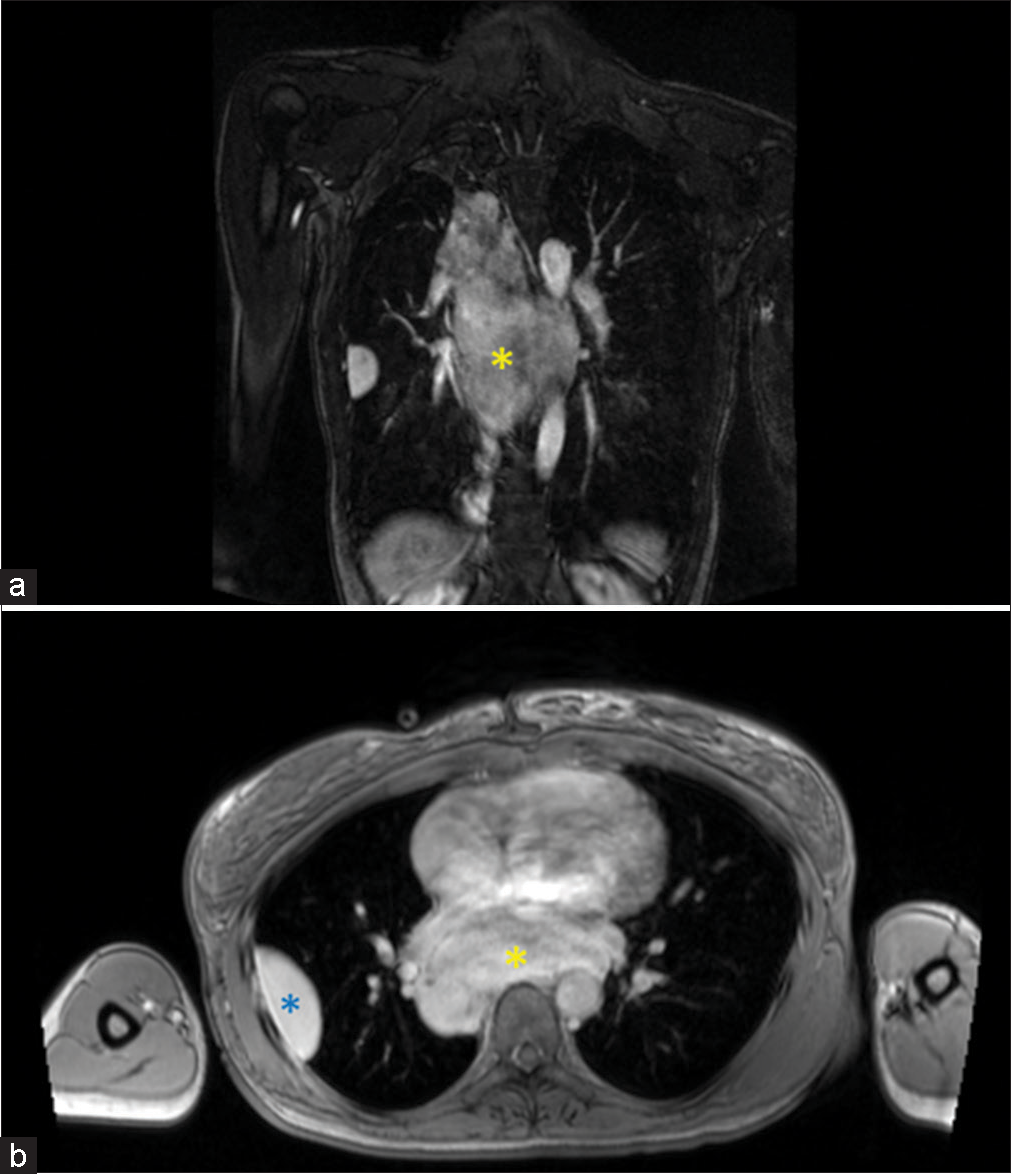
Figure 3:: A 65 years old who presented with chest pain and dyspnea. (a) Coronal T1 post-contrast magnetic resonance angiography sequences showing a large posterior mediastinal mass (yellow asterisk). (b) Axial T1 post-contrast image confirming the large posterior mediastinal mass (yellow asterisk) and a pleural-based mass (blue asterisk).
Export to PPT
The predominant and hallmark symptoms displayed by patients presenting to our ED were shortness of breath, chest pain, and cough (22/55, 40%). Patients presenting with dizziness, vomiting, or status epilepticus (3/55, 5.5%) were atypical non-specific symptoms. These symptoms are highly suggestive of an underlying pathology not associated with PE. Based on the data presented in Table 4, our radiologists’ ability to correctly discern PE was not affected by the imaging sequence. This is due to the fact that the imaging sequence depends on the type of MR scanner. Whether a patient was scanned with a bolus chase (33/55, 60%) or time-resolved MRA sequences (22/55, 40%), it did not appear to affect the diagnostic potential. However, current research supports that TWIST sequence is generally the preferred imaging sequence for acute indications due to time constraints, disoriented, and possibly hemodynamically compromised patients. Both imaging acquisition sequences played a key role in diagnosing PE in patients, even though MRA itself is a nonconventional technique.
The main imaging quality issue we noted in (44/55, 80%) of all scans was motion. Nonetheless, there was no statistically significant association between the initial complaints and quality issues. Motion is an unavoidable phenomenon most commonly owed to respiratory, cardiac, gastrointestinal, vessel pulsation, blood, CSF flow, and the long duration of image acquisition. This affects the image quality of vascular opacifications.[8,13] Motion does not appear to have negatively impacted the outcome of the MRA results, as respiratory self-gating technique was incorporated into these studies for both MR scanners where ED patients are imaged. Other techniques such as breath holding, k-space view reordering, and respiratory navigation techniques exist. Respiratory self-gating has proven essential, as its primary advantages lie in imaging severely ill or sedated patients who may have difficulty following with breath hold commands.[14] According to Grimm et al., there is reduction in motion blur and improved quantification accuracy compared to static reconstructions and in higher signal-to-noise ratio compared to conventional gated reconstructions.[15] Quality did not affect imaging interpretation nor subsequent patient management, thus resulting in a comparable outcome for patients.
The associations we found between opacification quality and initial complaint are likely due to the hallmark symptoms associated with suspicion of PE, mainly shortness of breath, chest pain, and cough were distributed among the 1 and 2 categories. This reflects the most optimal vessel opacification scores. Optimal vascular opacification is integral for pinpointing a PE diagnosis through demonstration of intravascular filling defects. The key symptoms with their corresponding Likert scale scoring recognize that dyspnea and/or chest pain are often present in 97% of patients with proven PE.[16] The correlation among these symptoms and Likert scale is important since MRA is not a conventional diagnostic tool. Most of the scans acquired were of high quality for diagnostic accuracy and with the combination of the radiologists’ expertise, we were less likely to misdiagnose an acute condition. This diagnostic efficacy is possible to due to recent advances in MR imaging technology, such as widespread 2D parallel imaging and improved contrast bolus injection protocols.[3] A predominant distribution of data (38/55, 69%) in the good to fair categories along with the radiologists’ expertise assure that accurate diagnoses reads were obtained. Patients presenting with dizziness, vomiting, or status epilepticus were suggestive of suffering from an alternate acute condition. In correspondence, these symptoms were distributed across the fair to poor vessel opacification. Among the good to fair distribution, two patients were found to have PE. One patient had a segmental PE and another had both lobar and segmental PE, as shown in Figures 4 and 5, respectively.
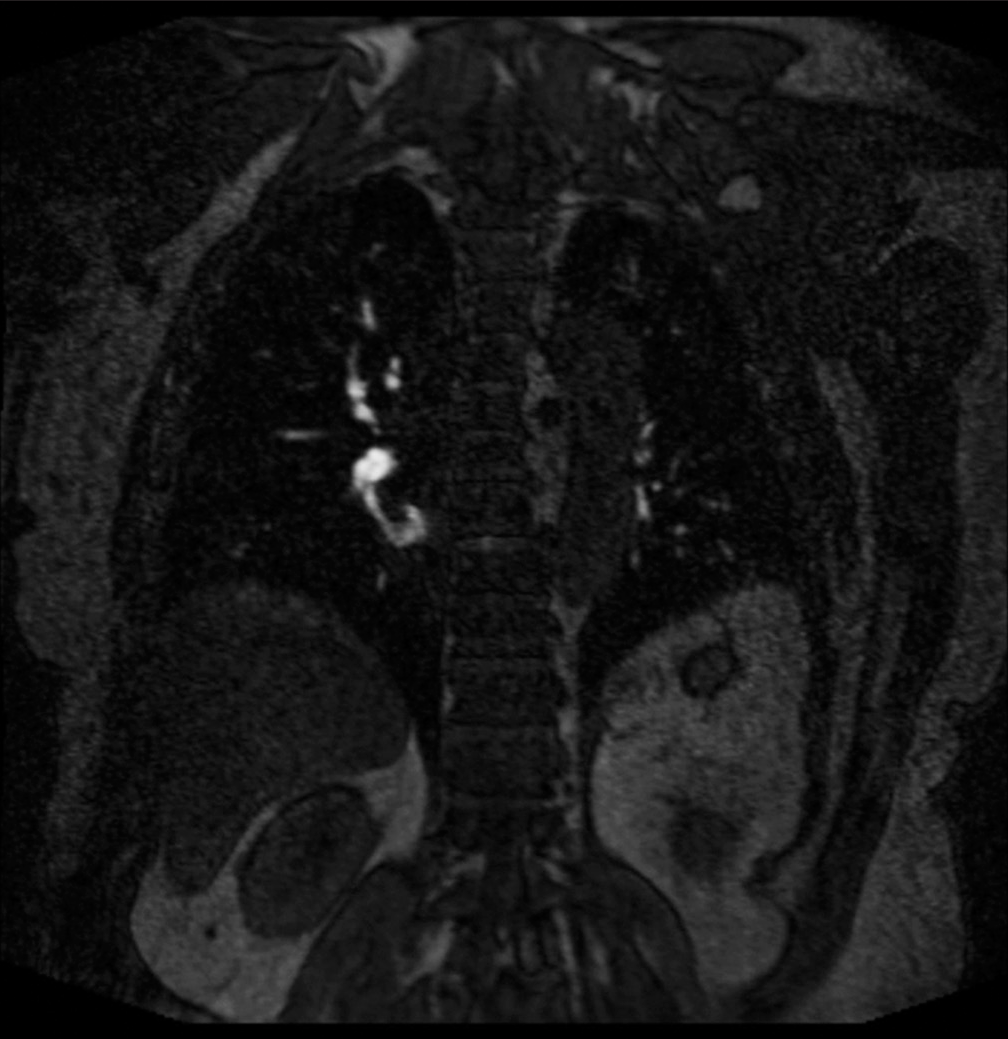
Figure 4:: A 69 year old who presented with chest pain and dyspnea. (a) Pulmonary embolus. (b) Coronal (TWIST) post-contrast T1 magnetic resonance angiography sequence showing a non-occlusive scant filling defect in the right lower lobe (red arrow). TWIST: Time-resolved angiography with stochastic trajectories.
Export to PPT
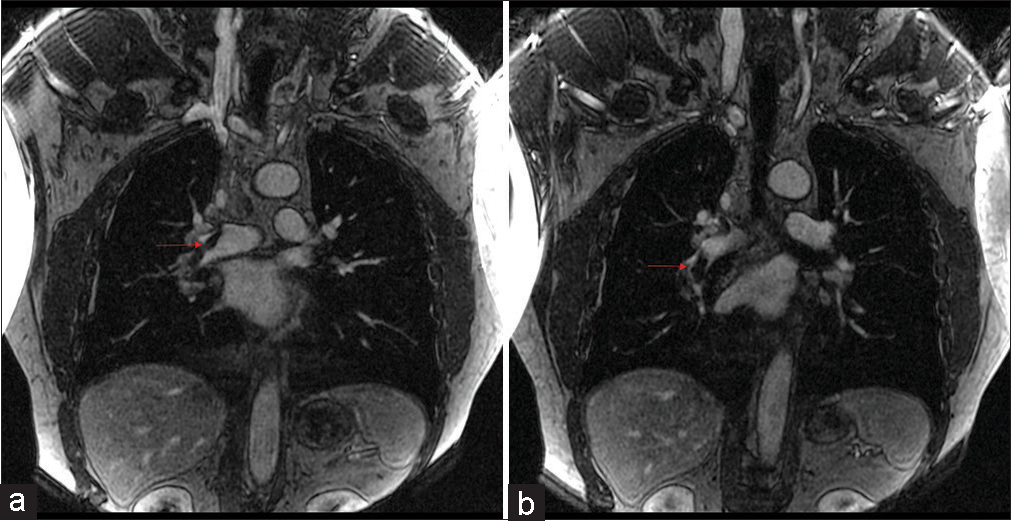
Figure 5:: A 60 years old who presented with chest pain and dyspnea. (a) Pulmonary embolus. (b) Coronal (TWIST) post-contrast T1 magnetic resonance angiography sequence showing a non-occlusive scant filling defect in the right lower lobe (red arrow). TWIST: Time-resolved angiography with stochastic trajectories.
Export to PPT
Our imaging results were predominantly negative. Based on multiple related articles, the consensus is that PE is a rare acute event and despite suspicion in patients that exhibit known symptoms, a majority of cases are often negative. The institution’s numbers are consistent with the annual national PE incidence of 39–115 cases/100,000 persons/ year.[17] According to Tsuchiya et al., MRA has a negative predictive value (NPV) of 99% which is similar to the NPV of CTA.[2] These facts are in line with our radiologists’ ability to accurately identify (53/55, 96.3%) of the imaging examinations as negative. Our results are parallel with Harringa et al.’s conclusion of 91.1% or more suspected PE cases are negative.[18] Pretest probability of PE for each patient was determined using the Wells criteria. As we found no significant associations between the initial complaint and risk score, we propose that clinical evaluation alone is not sufficient for effectively ruling out suspicion of PE in symptomatic ED patients. Finally, our data suggest Wells criteria may not accurately rule out PE, since its respective scoring can vary among clinicians and among institutions.[19]
There were several limitations to our study. First, it was a retrospective study at a single institution. Our sample size was relatively low at 55 over a period of only 2 months as this was the period of contrast shortage. Second, we were unable to compare the patient’s MRA images with a corresponding CTA examination since all the patients underwent an MRA scan only and although we verified in their EMR whether they were readmitted to the ED for similar symptoms up to 4 weeks after discharge, we could not track their long-term care. Third, there were only (7/55, 12%) patients exhibiting atypical symptoms of PE and these scored in the fair to poor range of the Likert scale which can imply that not all patients with atypical symptoms will always correspond with a poor Likert score. Finally, we had only two positive results and 53 negative results, so we were unable to calculate sensitivity, specificity, and negative and positive predictive values. Despite these limitations, our positivity rate of (2/55, 3.63%) is comparable to the national levels of CTA-identified PE. Kline et al. state that the positivity rate of PE across EDs throughout the country ranges from 0% to 18.9% with an overall rate of 6.9%.[20]
CONCLUSIONData from the contrast shortage period at our institution suggest that patients presenting to the ED with a high suspicion of PE can be evaluated through contrast-enhanced MRA as a second-tier alternative to CTA, but further investigation would be needed to verify and quantify the implications of not routinely giving intravenous contrast. In addition, the image quality of our MRA scans was ideal and our radiologists were able to consistently report the anatomic level of PE, which guided the treating ED physician for further steps in patient management. However, we should note that further = direct comparisons of MRA with CTA in larger patient populations across different settings are required to increase validity of the current case study. Moreover, there are drawbacks to the routine use of MRA, such as a higher operating cost, requirement of longer scanning times, and relatively less availability for acute ED use. Therefore, routine use for alternative indications would also prove unsustainable in the longer term and its utility in these contexts is thus relegated to emergent scenarios, such as the iodinated contrast shortage period from which our case study is based. In similar contexts, we support implementation of similar alterative imaging protocols so that large institutions such as ours, can respond to future crises, and decrease the likelihood of negative patient outcomes.
留言 (0)