Treadmill exercise exerts ameliorating effect on isolation-induced depression via neuronal activation
AbstractMaternal isolation has been used as a valid animal model of early life stress, and it induces depression to offspring. Exercise ameliorates the incidence and severity of stress-related mood disorders, such as depression and anxiety. Here in this study, we investigated the effects of treadmill exercise on brain neuronal excitation in the rat pups with maternal isolation-induced depression. Forced swimming test and immunohistochemistry for glucocorticoid receptor and c-Fos in the hippocampal dentate gyrus and hypothalamic paraventricular nucleus were conducted. Maternal isolation lasted for 6 hours per day and was continued from postnatal day 1 to postnatal day 30. The rat pups in the exercise group were forced to run on a treadmill for 30 min once a day for 10 consecutive days, starting from the postnatal day 21 until the postnatal day 30. In the present results, treadmill exercise alleviated depressive state in the maternal separated rat pups, as potently as fluoxetine treatment. Treadmill exercise also restored the expressions of glucocorticoid receptor and c-Fos in the hippocampal dentate gyrus and hypothalamic paraventricular nucleus of the maternal separated rat pups near to the control level, as fluoxetine treatment. The present study suggests the possibility that treadmill exercise can be used as the therapeutic strategy for the childhood depression induced by disturbed mother-child relationship.
Keywords: Maternal isolation, Treadmill exercise, Glucocortcoid receptor, c-Fos, Hippocampal dentate gyrus, Hypothalamic paraventricular nucleus
INTRODUCTION Stress is a jargon used to describe experiences that are challenging emotionally and physiologically. Early adverse events enhance risks of post-traumatic stress disorder, depression, and addiction in the adulthood (Heim and Nemeroff, 2001; Yehuda et al., 2001). Stress in early life elicited long-term changes in the multiple neurotransmitter systems and brain structures, and these neurobiological changes may underlie the increased vulnerability to neuropsychiatric disorders in later life (Avitsur and Sheridan, 2009; Kaufman et al., 2000). Stress acts through the actions of glucocorticoids, that is closely implicated in the depressive illness, and exerts profound effects on brain functions (Yu et al., 2008). Corticosteroids (corticosterone in rodents and cortisol in man) in blood easily cross the blood brain barrier and can the bind to glucocorticoid receptor (GR) in the brain (Reul and de Kloet, 1985; Yi et al., 2009;). In the rodent brain, the GR is widely distributed and particularly dense in the hippocampus and in the hypothalamic paraventricular nucleus (PVN) (Sánchez et al., 2000). The brain is a target of stress, and the hippocampus is the most sensitive brain to the glucocorticoids, besides the hypothalamus (McEwen, 2007). Stress down-regulates GR and its mRNA expression (Ladd et al., 2004; Uys et al., 2006; Workel et al., 2001). Also, glucocorticoids modulate stress responses via negative feedback in the pituitary gland, hypothalamus, and limbic area (Yi et al., 2009). Glucocorticoids cause their effects by binding to the GR which are found in the cells of almost all vertebrate tissues (Kassel and Herrlich, 2007). Downregulation or deletion of GR has been considered as the important factor inducing anxiety and depressive behaviors (Chiba et al., 2012; Solomon et al., 2012). Early adverse experiences resulting from maternal isolation may eventually cause memory impairment (Baek et al., 2012; Sung et al., 2010). Repeated maternal isolation has been used as the animal model of depression in offspring (Elizalde et al., 2008; Sung et al., 2010). Treadmill exercise has commonly recommended to alleviating stress-induced depression and its complications, however, the effect of treadmill exercise on the glucocorticoids in relation with neuronal activity in the disturbed mother-child relation-induced depression has not yet been clarified. In the present study, the effect of treadmill exercise on GR and c-Fos expressions in the rat pups with maternal isolation-induced depression were investigated. Forced swimming test and immunohistochemistry for GR and c-Fos in the hippocampal dentate gyrus and hypothalamic paraventricular nucleus were conducted. MATERIALS AND METHODS Animals and treatmentsMale Sprague-Dawley rats (250±10 g, 12 weeks old) and female Sprague-Dawley rats (180±10 g, 8 weeks old) were used in this study. Female rats (n=5) were allowed to mate with male rats (n=10) for 24 h. One day later, the female rats were separated from the male rats and housed individually in a plastic home cage at the controlled temperature (20±2°C) and the light-dark cycle of 12 h of light and 12 h of darkness (light on from 07:00 to 19:00 h). Food and water were made available ad libitum. The experimental procedures were performed in accordance with the animal care guidelines of National Institutes of Health (NIH) and Korean Academy of Medical Sciences.
The day of delivery was designated postnatal day 0. After delivery, each of rat pups from each dam was assigned into one of 4 groups (n=8): the control group, the maternal isolation group, the maternal isolation with treadmill exercise group, the maternal isolation with fluoxetine administration group.
Maternal isolation On postnatal day 1, the rat pups were labeled by oily marker. Maternal isolation lasted for 6 h per day and was continued from postnatal day 1 to postnatal day 30, as the previously described method (Sung et al., 2010). The isolation procedure was performed by removing all pups from their home-cage and placing them into the separated clean cage. Following maternal isolation for 6 h, the rat pups were returned to their home cage and cared by their dam. Treadmill exercise protocol and fluoxetine administration The rat pups in the exercise group were forced to run on a treadmill for 30 min once a day for 10 consecutive days, starting from the postnatal day 21 until the postnatal day 30. The exercise load consisted of running at a speed of 2 meters/min with the 0° inclination. This exercise regimen is a light-intensity exercise (Kim et al., 2003).The rat pups in the fluoxetine administration group received 5 mg/kg fluoxetine hydrochloride (Tocris, Bristol, UK) orally once a day from the postnatal day 21 until the postnatal day 30. The rat pups in the other groups received same amount of distilled water for the same duration.
Forced swimming test In order to evaluate the depressive state of the rat pups, forced swimming test was conducted as the previously described method (Baek et al., 2012; Khisti et al., 2000). On postnatal day 29, the rat pups were exposed to a pre-test for 15 min., and then 24 h after, they were exposed test session for 4 min. The animals were placed individually in a vertical plexiglas cylinder (height: 45 cm, diameter: 19 cm) filled with 30 cm of 26±1°C water. During the test session, the immobility was analyzed using Smart version 2.5 video tracking system (Panlab, Barcelona, Spain). Finally, the incidences of swimming and climbing were added together to represent “active behavior” score. Tissue preparationThe rat pups were sacrificed on postnatal day 30, after last exercise or fluoxetine administration. At the beginning of the sacrificial procedure, the animals were weighed and overdosed with Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France). After a complete lack of response was observed, the rat pups were transcardially perfused with 50 mM phosphate-buffered saline (PBS), and then with 4% paraformaldehyde in 100 mM phosphate buffer (PB) at pH 7.4. The brains were dissected, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Serial coronal sections of 40 μm thickness were made using a freezing microtome (Leica, Nussloch, Germany).
GR immunohistochemistry For the evaluation of the optical density of GR in the hippocampal dentate gyrus and the number of GR-positive cells in the hypothalamic paraventricular nucleus, GR-specific immunohistochemistry was performed as the previously described method (Park et al., 2005). Five sections on average were selected in each brain region spanning from Bregma −3.3 mm to −4.16 mm. Free-floating tissue sections were incubated overnight with rabbit anti-GR antibody (Santa Cruz Biotechnology, Santa, CA, USA) at a dilution of 1:500 for visualization of GR distribution. Then, the sections were incubated for 1 h with anti-rabbit secondary antibody (1:200, Vector Laboratories, Burlingame, CA, USA). The sections were subsequently incubated with an avidin-biotin-peroxidase complex (1:100, Vector Laboratories) for 1 h at room temperature. Immunoreactivity was visualized by incubating the sections in a solution consisting of 0.02% 3,3-diaminobenzidine (DAB) and 0.03% H2O2 in 50 mM Tris-HCl (Ph 7.6) for approximately 5 min. Then the sections were mounted on gelatincoated glass slides. The slides were air-dried overnight at room temperature, and coverslips were mounted using Permount® (Fisher Scientific Co, Pittsburgh, PA, USA). c-Fos immunohistochemistry c-Fos immunohistochemistry was performed as the previously described method (Kim et al., 2012b). The sections were collected in 0.1 M PBS and subsequently processed free-floating according to the avidin-biotin method. The tissue sections were incubated overnight with rabbit anti-Fos antibody (Santa Cruz Biotechnology) at a dilution of 1:1,000, and the sections were then incubated for 1 h with biotinylated anti-rabbit secondary antibody (Vector Laboratories). The sections were subsequently incubated with avidin-biotin-peroxidase complex (Vector Laboratories) for 1 h at room temperature. Immunoreactivity was visualized by incubating the sections in a solution consisting of 0.05% DAB and 0.01% H2O2 in 50 mM Tris-buffer (pH 7.6) for approximately 3 min. The sections were then washed three times with 0.1 M PBS and mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and coverslips were mounted using Permount® (Fisher Scientific Co.). As the negative control, the brain sections were likewise processed using normal goat serum in place of the primary antibody, and no c-Fos-like immunoreactivity was observed. Data analysesThe area of the granular layer of the hippocampal dentate gyrus and hypothalamic paracentricular nucleus was measured using Image-Pro®Plus image analyzer (Media Cybernetics Inc., Silver Spring, MD, USA). The optical density of GR in the hippocampal dentate gyrus eas measured using Image-Pro®Plus image analyzer (Media Cybernetics Inc.). The number of GR-positive-cells in the hypothalamic paraventricular nucleus and the numbers of c-Fos-positive cells in the hippocampal dentate gyrus and in the hypothalamic paraventricular nucleus were counted hemilaterally, and expressed as the number of cells per mm2 of the cross-sectional area. All data were analyzed using the statistical software SPSS (version 17.0). The data are expressed as the mean±standard error of the mean (SEM). For the comparison among the groups, oneway ANOVA and Tukey HSD post-hoc test were performed and differences were considered statistically significant at P<0.05.
RESULTS Effect of treadmill exercise on the depressive state in the forced swimming test The sum of swimming and climbing time in the force swimming test was 61.12±16.54 sec in the control group, 27.69±16.31 sec in the maternal isolation group, 61.35±26.85 sec in the the maternal isolation with treadmill exercise group, and 81.78±32.09 sec in the the maternal isolation with fluoxetine administration group (Fig. 1). The present results indicated that the maternal separated rat pups showed decreased sum of swimming and climbing time compared to the control rat pups (PP Effect of treadmill exercise on the expression of GR in the hippocampal dentate gyrus The mean optical density of GR-positive cells in the hippocampal dentate gyrus was 80.79±4.91 in the control group, 72.74±5.21 in the maternal isolation group, 83.75±6.75 in the maternal isolation with treadmill exercise group, and 81.63±2.20 in maternal isolation with fluoxetine administration group (Fig. 2). The present results indicated that the maternal separated rat pups showed decreased density of GR in the hipppocampal dentate gyrus compared to the control rat pups (PP Effect of treadmill exercise on the c-Fos expression in the hippocampal dentate gyrus The mean number of c-Fos-positive cells in the hipppocampal dentate gyrus was 520.95±107.44/mm2 in the the control group, 233.38±95.58/mm2 in the maternal isolation group, 434.40±96.25/mm2 in the maternal isolation with treadmill exercise group, and 412.08±150.79/mm2 in the maternal isolation with fluoxetine administration group (Fig. 3). The present results indicated that the maternal separated rat pups showed decreased number of c-Fos-positive cells in the hipppocampal dentate gyrus compared to the control rat pups (PP Effect of treadmill exercise on the GR expression in the hypothalamic paraventricular nucleus The mean number of GR-positive cells in the hypothalamic paraventricular nucleus was 355.58±119.50/mm2 in the control group, 239.47±115.91/mm2 in the maternal isolation group, 367.40±92.25/mm2 in the maternal isolation with treadmill exercise group, and 362.67±118.78/mm2 in the maternal isolation with fluoxetine administration group (Fig. 4). The present results indicated that the maternal separated rat pups showed decreased number of GR-positive cells in the hypothalamic paraventricular nucleus compared to the control rat pups (PP Effect of treadmill exercise on the c-Fos expression in the hypothalamic paraventricular nucleus The mean number of c-Fos-positive cells in the hypothalamic paraventricular nucleus was 110.04±28.32/mm2 in the control group, 74.70±18.57/mm2 in the maternal isolation group, 136.82±72.36/mm2 in the maternal isolation with treadmill exercise group, and 128.50±37.29/mm2 in the maternal isolation with fluoxetine administration group (Fig. 5). The present results indicated that the maternal separated rat pups showed decreased number of c-Fos-positive cells in the hypothalamic paraventricular nucleus compared to the control rat pups (PP DISCUSSION Maternal isolation during early postnatal period increases vulnerability to neuropsychiatric disorders such as anxiety, personality disorders, and depression (Baek et al., 2012; Kalinichev et al., 2002; Sung et al., 2010). The present study investigated whether maternal isolation for 6 h per day in the early life stage induces depressive state with functional alteration in the hippocampus and hypothalamus. The effect of treadmill exercise on the depressive state induced by maternal isolation was also compared with the antidepressant fluoxetine. Forced swimming test is one of the most suitable tests evaluating depressive state in animals (Baek et al., 2012; Kim et al., 2012a). Forced swimming test is also widely used to screen compounds having antidepressant properties (Reed et al., 2008). Antidepressant treatments increased active escape behaviors, such as climbing or swimming, to escape from the stressful situation (Cryan et al., 2005). Decrease in climbing time and increase in immobility time indicates depressive state of the rats (Kim et al., 2012a). In the present study, repeated maternal isolation during neonatal period decreased time of swimming and climbing in the rat pups, representing that maternal isolation induced depressive state in the rat pups. Treadmill exercise and fluoxetine treatment increased this time, representing that treadmill exercise and fluoxetine treatment alleviated depressive state in the maternal separated rat pups. Previous studies demonstrated that stressed rat pups exhibited immobilization activity after postnatal maternal isolation experience (Aisa et al., 2008; Slotten et al., 2006). In contrast, anti-depressive treatment increased active behavior in the maternal isolation rat pup (Rokni-Yazdi et al., 2007).In the present study, maternal isolation reduced the density of GR in the hippocampal dentate gyrus and the number of GR in the hypothalamic paraventricular nucleus of the maternal isolation rat pups. Treadmill exercise and fluoxetine treatment increased the density of GR in the hippocampal dentate gyrus and the number of GR-positive cells in the hypothalamic paraventricular nucleus of the maternal isolation rat pups.
The animals exposed to early life trauma showed an increase in basal corticosterone levels, and a significant decrease in the ratio of GR-positive cells to total cells in the hilus, granule cell layer, and dentate gyrus (Uys et al., 2006). The number of GR-positive cells in the paraventricular nucleus of hypothalamus was decreased by acoustic trauma in mice (Tahera et al., 2007). As the effects of glucocorticoids are mediated by their receptors, the number and/ or function of GR were reduced in the depressive patients (Yu et al., 2008). Chronic subordinate colony (CSC) housing, validated as a murine model of chronic psychosocial stress, decreased the number of GR in mice, similar to our results (Singewald et al., 2009).In the present study, maternal isolation reduced the numbers of c-Fos-positive cells in the hippocampal dentate gyrus and in the hypothalamic paraventricular nucleus of the rat pups. Treadmill exercise and fluoxetine treatment increased the numbers of c-Fos-positive cells in the hippocampal dentate gyrus and in the hypothalamic paraventricular nucleus of the maternal isolation rat pups.
Maternal isolation suppressed basal ornithine decarboxylase g ene transcription in the neonatal pups through downregulation of ornithine decarboxylase gene transactivator proto-oncogenes c-myc and max (Wang et al., 1996). Maternal behavior promoted cellular activity that induced neuronal expression of c-fos in many forebrain sites (Lonstein et al., 1998). c-Fos is an immediate early gene and its expression is sometimes used as a marker for stimulus-induced changes in the metabolic activity of neurons. Jang et al. (2003) suggested that alcohol-induced decrement of the number of Fos-positive cells in the hippocampus represents disruption of hippocampal functions. Baek et al. (2006) reported that maternal isolation significantly suppressed c-Fos expression in the hippocampus of the rat pups and that treadmill exercise enhanced c-Fos expression in the hippocampus of the maternal separated rat pups. They suggested that treadmill exercise may be a useful strategy to prevent or cure those who suffer from decreased neuronal activity induced by maternal isolation in childhood (Baek et al., 2006). Intraperitoneal injection of cyclophosphamide increased c-Fos expression in the micturition centers, such as the pontine micturition center, ventrolateral periaqueductal gray, medial pre-optic nucleus, and spinal cord, indicated that induction of overactive bladder activated neurons in the voiding centers (Kim et al., 2012b). Hypothalamic-pituitary-adrenal (HPA) dysregulation is a key feature of affective disorders. The influence of the limbic system on the HPA axis is likely the end result of the overall patterning of responses to given stimuli and glucocorticoids, with the magnitude of the secretory response determined with respect to the relative contributions of the various structures (Herman et al., 2005). Postnatal maternal isolation increased HPA and behavioral responses to stress, in contrast, environmental enrichment led to the functional reversal of the effects of maternal isolation (Francis et al., 2002). Prolonged stress causes dysregulation of the HPA axis and may contribute to the pathogenesis of major depressive disorder (Becker et al., 2009). As dysfunctional GR-mediated negative feedback regulation of cortisol levels is implicated in over-activity of the HPA axis, agents that intervene with the mechanisms involved in dysregulation of cortisol synthesis and release are under investigation as possible therapeutic agents (Thomson and Craighead, 2008). Running, such as a wheel running paradigm, is a natural rodent behavior that seems to have a hedonic factor (Stranahan et al., 2006). Exercise decreased depressive-like behavior through increasing brain-derived neurotrophic factor (BDNF) expression in maternal separated rats (Marais et al., 2009). Quantitative proteomic analysis revealed that several proteins, involved in neuronal structure, metabolism, signalling, anti-oxidative stress, and neurotransmission, were altered by maternal isolation, that many of these proteins were restored to normal by subsequent exposure to voluntary exercise in adolescence (Daniels et al., 2012). Baek et al. (2012) demonstrated that postnatal treadmill exercise alleviated maternal isolation-induced depressive state by suppressing apoptotic neuronal cell death in the hippocampus.Decreased GR and c-Fos expressions in the maternal separated rat pups suggests that maternal isolation induced dysregulation of HPA axis with inhibited neuronal activity, resulted in depressive state of the rat pups. Treadmill exercise alleviated maternal isolation-induced depressive state through enhancing of GR and c-Fos expressions, as potent as fluoxetine treatment. The present study suggests the possibility that treadmill exercise can be used as the therapeutic strategy for the depression induced by disturbed mother-child relationship.
ACKNOWLEDGMENTSThis work was supported by the Incheon National University Research Grant in 2012.
REFERENCES Aisa B, Tordera R, Lasheras B, Del Río J, Ramírez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–1226.The effect of treadmill exercise on the time of swimming and climbing in the forced swimming test. (A) Control group, (B) maternal isolation group, (C) maternal isolation with treadmill exercise group, (D) maternal isolation with fluoxetine administration group. a)Represents P< 0.05 compared to the control group. b)Represents P< 0.05 compared to the maternal isolation group. Values are represented as the mean± standard error of the mean (SEM).
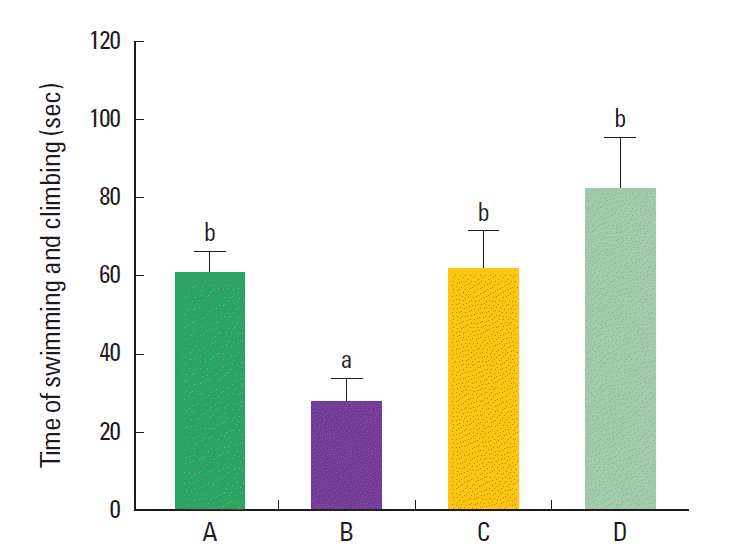 Fig. 2
Fig. 2
The effect of treadmill exercise on the density of glucocorticoid receptor (GR) in the hippocampal dentate gyrus. (A) Photomicrographs of GR-expressing cells. The scale bar represents 25 μm. (B) Mean optical density of GR-positive cells in each group. (A) Control group, (B) maternal isolation group, (C) maternal isolation with treadmill exercise group, (D) maternal isolation with fluoxetine administration group. a)Represents P< 0.05 compared to the control group. b)
Comments (0)