PURPOSE We aimed to assess long-term inhaled corticosteroid (ICS) risks in chronic obstructive pulmonary disease (COPD) management.
METHODS We extracted electronic health record data for individuals aged >45 years with COPD from a data repository. The prevalent cohort required a diagnosis of COPD any time during the observation period, and the inception cohort required a diagnosis of COPD made after entry into the database. A composite outcome of any new diagnosis of type 2 diabetes, cataracts, pneumonia, osteoporosis, or nontraumatic fracture; and recurrent event outcomes of repeated pneumonia or nontraumatic fracture were compared for long-term (>24 months) vs short-term (<4 months) ICS exposure.
RESULTS We assessed outcomes for 318,385 and 209,062 individuals in the prevalent and inception cohorts, respectively. The composite dichotomous outcome was significantly greater for long-term vs short-term ICS use for the prevalent (hazard ratio [HR] = 2.65; 95% CI, 2.62-2.68; P <.001) and inception (HR = 2.60; 95% CI, 2.56-2.64; P <.001) cohorts. For the inception cohort, the absolute risk difference of the composite outcome was 20.26% (29.41% minus 9.15%), with a number needed to harm of 5. Hazard ratios were significantly increased in the prevalent and inception cohorts for recurrent pneumonia (HR = 2.88; 95% CI, 2.62-3.16; P <.001 and HR = 2.85; 95% CI, 2.53-3.22; P <.001, respectively) and recurrent fracture (HR = 1.77; 95% CI, 1.42-2.21; P <.001 and HR = 1.57; 95% CI, 1.20-2.06; P <.001).
CONCLUSIONS Long-term ICS use for COPD is associated with significantly greater rates of the composite outcome of type 2 diabetes, cataracts, pneumonia, osteoporosis, and nontraumatic fracture; recurrent pneumonia; and recurrent fracture.
Key words:INTRODUCTIONChronic obstructive pulmonary disease (COPD) is the sixth leading cause of death in the United States and the fourth worldwide as of 2024.1,2 More than 16 million people in the United States have been diagnosed with COPD.3 Current recommendations for COPD pharmacotherapy4 list inhaled long-acting bronchodilators as first-line maintenance therapy, with preference for long-acting muscarinic antagonists (LAMAs) alone or in combination with long-acting beta-adrenoceptor agonists (LABAs).5-7 Yet, the most commonly used first-line COPD maintenance therapy is the combination of an inhaled corticosteroid (ICS) with a LABA.8-10
Use of an ICS is only considered first-line therapy for people with COPD who also have concomitant asthma or asthma-like features, termed asthma/COPD overlap (ACO), or have high total eosinophil counts.11 Fewer than 25% of those with a COPD diagnosis also have asthma or ACO.11 For COPD without ACO, an ICS is indicated for the prevention of frequent moderate to severe exacerbations not responsive to other treatments for individuals with high blood eosinophil counts.6,12,13 Thus, many individuals with COPD are regularly using an ICS, which might not be indicated or recommended for their level of COPD.4 For this group, the risks of long-term ICS therapy14-17 must be balanced with the limited benefits of ICS.
Long-term risks of >2 years of ICS exposure include increased risks of osteoporosis and fractures,17-19 hyperglycemia and new-onset diabetes,17,20,21 cataract formation and glaucoma,22 pneumonia,23,24 and rare episodes of adrenal insufficiency.23-25 Few studies have evaluated long-term ICS risks in US populations of individuals with COPD. Here, we used electronic health record (EHR) data to examine long-term ICS exposure risks in a large cohort of patients with COPD.
METHODSWe combined the DARTNet Practice Performance Registry26 and subsets of previous research study cohorts to create a database of EHR data standardized to the Observational Medical Outcomes Partnership Common Data Model (https://www.ohdsi.org/data-standardization). The resultant database included more than 20 million individuals with a look-back period of up to 12 years. We extracted a subset of EHR data from individuals >45 years of age with a diagnosis of COPD (see Supplemental Table 1 for full code list). Data elements included demographic characteristics, medical encounters, conditions, medications, and selected measurements and procedures. The original COPD cohort and subsequent data cleaning steps are shown in Figure 1.
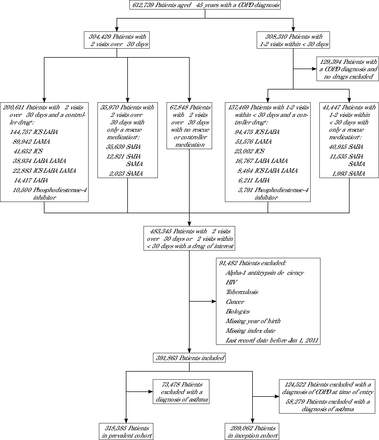
 Figure 1.
Figure 1. STROBE Diagram
COPD = chronic obstructive pulmonary disease; ICS = inhaled corticosteroid; LABA = long-acting beta-adrenoceptor agonist; LAMA = long-acting muscarinic antagonist; SABA = short-acting beta-adrenoceptor agonist; SAMA = short-acting muscarinic antagonist; STROBE = Strengthening the Reporting of Observational Studies in Epidemiology.
a Categories not mutually exclusive; 1 patient can be listed in >1 medication category.
InclusionThe final cohorts used for analysis consisted of a prevalent cohort, which included all individuals with a database diagnosis of COPD at any time during the observation period including at entry into the database, and an inception cohort, in which a first diagnosis of COPD was recorded after ≥6 months or 2 documented visits in the database. For both cohorts, a diagnosis of COPD required ≥2 visits ≥30 days apart, with diagnosis codes indicating COPD/chronic bronchitis or COPD exacerbation, or 1 visit and 1 prescription for COPD maintenance or rescue therapy, or 1 hospitalization for COPD. For all individuals in both cohorts and all study outcomes, the time period for the end of analysis was the latest date in the database of some indication of an interaction with the health system (eg, a visit, laboratory test, telephone call, refill, or procedure). Thus, all individuals were alive at the end of their analytical time period. No death data were used.
ExclusionIndividuals were excluded from analysis if they had at any time a diagnosis of (1) tuberculosis or nontuberculous mycobacteria; (2) idiopathic lung disease/fibrosis/alpha-1 anti-trypsin deficiency; or (3) HIV/AIDS. Individuals were also excluded if there was any evidence of active treatment for any malignancy other than basal or squamous cell skin cancer, use of biologic treatment for any long-term or chronic condition, and long-term use of oral steroids (daily or every other day use for ≥3 consecutive months). Primary analyses also excluded individuals with a concomitant diagnosis of asthma; COPD inception and prevalent cohorts with concomitant diagnoses of asthma were evaluated as a sensitivity analysis.
Inhaled Corticosteroid ExposureInhaled corticosteroid exposure was categorized into the following 3 groups: (1) ICS alone or plus any combination of LAMA, LABA, short-acting beta-adrenoceptor agonist or short-acting muscarinic antagonist for >24 months (long-term exposure); (2) ICS alone or any above combination for 4-23 months (intermediate-term exposure); (3) ICS alone or any above combination for <4 months (short-term ICS exposure). Exposure to phosphodiesterase-4 inhibitor or maintenance antibiotics (typically azithromycin) was allowed in any group. Intermediate-term results are shown in Supplemental Tables 2 and 3.
OutcomesThe primary outcomes were (1) a single event composite outcome of a new diagnosis of type 2 diabetes, cataracts, pneumonia, osteoporosis, or nontraumatic fracture; and (2) recurrent event outcomes of repeated pneumonia or repeated nontraumatic fracture, with comparisons made between the short-term ICS group and the long-term ICS group. Secondary outcomes were univariate comparisons between all groups. We examined each individual outcome independently to ensure the correct directionality of the outcome between the short-term ICS group and the long-term ICS group before inclusion in the composite final outcome. Only correct directionality and not statistically significant differences were required for inclusion of an outcome in the composite primary outcome.
Type 2 diabetes was considered new if there was no prior diagnosis within 6 months or within 2 encounters from entry into the database plus any of the following: 2 diagnoses of type 2 diabetes <18 months apart, a diagnosis of type 2 diabetes and treatment with a diabetes medication, a hemoglobin A1c level >6.4%, a fasting glucose level of >124 mg/dL, or a random glucose level of >250 mg/dL.27 Pneumonia was considered new if no pneumonia diagnosis was present in the prior 6 months or 2 visits, or considered a repeat pneumonia if diagnostic codes occurred ≥60 days apart from the initial diagnosis. A new diagnosis of osteoporosis and a new-onset nontraumatic fracture required the same 6-month or 2-visit criteria before first diagnosis. Multiple nontraumatic fractures were only considered new if they involved a different bone than any previous fracture. Cataracts were considered a single event and were included based on cataract diagnostic or surgical procedure codes.
AnalysesTo address nonrandom assignment of individuals to any of the ICS exposure groups, we engaged in propensity matching using genetic matching in R (R Project for Statistical Computing) with the exact matching algorithm.28,29 Genetic matching iteratively recalculates the propensity scores after each match to determine the next best match. The following covariates were used for propensity matching: sex, race, ethnicity, body mass index, smoking status, and Charlson-Deyo score dichotomized at >2.30,31 Matching was performed with 1:1, 2:1, and 3:1 groupings depending on the differences in sizes of the cohort pairs to use as many individuals as possible in each analysis. All analyses were performed on these propensity-matched cohorts. Results were also controlled for age and systemic steroid use in the overall model.
We assessed descriptive statistics for the unmatched cohorts and the matched cohorts. We completed univariate analyses using proportion tests for all cohort pairs (secondary outcome). Cox proportional hazards models were then run between the 6 different cohort pairs, prevalent and inception cohorts by short-term ICS exposure vs long-term exposure, short-term ICS exposure vs intermediate-term exposure, and intermediate-term exposure vs long-term exposure. We used Andersen-Gill models for repeated outcomes. Univariate analyses were run in R v4.3.1 (Beagle Scouts), and regressions were completed in SAS 9.4 (SAS Institute). We conducted sensitivity analyses for the prevalence and incidence cohorts for those with a concomitant asthma diagnosis. We also conducted sensitivity analyses for time to first event, total exacerbations vs systemic steroid bursts (primarily used for exacerbations), use of selected medications as a proxy for a diagnosis of type 2 diabetes, and use of bisphosphonate therapy as a proxy for a diagnosis of osteoporosis.
We selected an alpha level of .05 to determine significance, with a Bonferroni adjustment for multiple comparisons, statistical significance of .05/4 = 0.0125. Given the large sample size for these analyses, absolute percentage differences were also evaluated independently by 2 physician authors (W.D.P. and B.P.Y.) for clinical significance. The number needed to harm was calculated for the inception cohorts as follows: 100 ÷ (% of individuals in long-term cohort with ≥1 outcome minus % of individuals in short-term ICS group with ≥1 outcome × 100).
RESULTSOf the original 621,739 individuals with a COPD diagnosis in the database, after removing those with 1 COPD diagnosis with no prescribed medication, various respiratory comorbidities, and those with a diagnosis of asthma, 318,385 were available for matching in the prevalent cohort and 209,062 were available in the inception cohort. Figure 1 shows the Strengthening the Reporting of Observational Studies in Epidemiology diagram and the effect of various cohort requirements on the final analytic groups. The mean length of follow-up data across the entire population was 3.9 (SD 3.1) years. As expected, the matched subgroups were essentially identical for all variables applied (Supplemental Table 2). The demographic characteristics of the prevalent and inception cohorts are listed in Table 1. There were no clinically meaningful differences in age, sex, race, and ethnicity between the groups. Charlson-Deyo scores were greater in the inception vs prevalent cohort. Cohort demographic characteristics by ICS duration group are listed in Table 2.
Table 1.Demographic Characteristics of Prevalent and Inception Cohorts
Table 2.Cohort Demographic Characteristics by ICS Duration Group (Matched)
Table 3 presents the univariate frequency comparisons and analyses of individual primary outcomes. For the prevalent cohort, all individual outcome frequencies were significantly greater in the long-term vs short-term ICS group. The inception cohort findings were similar. Based on this, all individual outcomes were included in the final composite outcome.
Table 3.Univariate Analysis for Directionality, COPD Alone, and COPD + Asthma
The composite dichotomous outcome was significantly greater in the long-term ICS group vs the short-term ICS group for both the prevalent (hazard ratio [HR] = 2.65; 95% CI, 2.62-2.68; P <.001) and inception (HR = 2.60; 95% CI, 2.56-2.64; P <.001) cohorts (Table 4). A sensitivity analysis of time to event confirmed our primary analysis (Supplemental Figure 1). The absolute risk difference of 29.41% of individuals having ≥1 of the outcome events for the inception long-term ICS group compared with 9.15% for the inception short-term ICS group translates to a number needed to harm of 5. We performed a sensitivity analysis using all exacerbations in place of only steroid-containing exacerbations (87.8% of all exacerbations), with no change in the results. Sensitivity analyses including a bisphosphonate prescription as part of the osteoporosis phenotype did not change the results, nor did including selected medications to treat type 2 diabetes as part of that phenotype.
Table 4.Primary and Sensitivity Analysis Results of Cox Proportional Hazards Regression Models of Prevalent and Inception Cohorts
The HRs for the individual recurrent outcomes in the prevalent cohort were significantly greater for the long-term vs short-term ICS group for pneumonia (HR = 2.88; 95% CI, 2.62-3.16; P <.001) and fracture (HR = 1.77; 95% CI, 1.42-2.21; P <.001), with similar results for the inception cohort (pneumonia HR = 2.85; 95% CI, 2.53-3.22; P <.001 and fracture HR = 1.57; 95% CI, 1.20-2.06; P <.001). The sensitivity analyses that included those with an ever asthma diagnosis were robust to this change.
The secondary outcomes of the composite dichotomous comparison and the total number of pneumonia and fracture events between the intermediate-term exposure groups in the prevalent and inception cohorts and the long-term exposure groups were also significantly different (P <.001 for all 4 comparisons) (Supplemental Table 4).
DISCUSSIONThis study focused on the long-latency side effects of ICS in individuals with COPD. Most of the side effects investigated are well known to be increased with long-term systemic steroids but have not been as well delineated for long-term ICS use. The focus on individuals with COPD is particularly important because ICS therapy is not first-line maintenance therapy for this condition, yet it is often prescribed without apparent indication. Composite and univariate assessments indicate that longer-term ICS use significantly increases the overall risk of the composite of the untoward outcomes as well as each outcome individually. The combined side effects occurred in an additional 20.26% of individuals on long-term ICS therapy compared with those with COPD not on long-term ICS therapy, yielding a number needed to harm of 5. These differences were considered clinically significant by both physician authors who analyzed the data.
Our findings support and expand on previous studies indicating that long-term (>24 months) use of ICS to treat COPD increases the risk of type 2 diabetes, cataracts, pneumonia, osteoporosis, and nontraumatic fractures.17-21,32 Not all studies have found similar results. Ng et al did not find an increased risk of fracture in ICS users.33 That population was drawn from a bone-mineral density registry; thus, all individuals were being actively screened for osteoporosis, and 58.7% were on active therapy for osteoporosis. Combined with a relatively small sample size, the unique population could have led to the null outcome. Flynn et al assessed a cohort of 4,305 people (3,243 exposed to ICS) and did not find associations with new-onset diabetes or fractures.34 Mean exposure time was not reported. Propensity matching was used as a sensitivity analysis, and the control cohort was small. Pu et al conducted a meta-analysis of drug approval studies and did not find associations with our outcomes,35 which is not inconsistent with our results, given that those studies usually followed people for intermediate timeframes and thus were not able to address long-latency side effects. None of these studies evaluated the combined morbidity associated with ICS use across all outcomes.
There are likely multiple reasons for the high prevalence of ICS use in individuals with COPD in the United States (>60%)36 as a result of ICS/LABA being the most common maintenance therapy. Perhaps foremost, there appears to be a perceived low side-effect burden of corticosteroids used in this fashion. Given that only short-term side effects can be reported in typical 12- to 18-month efficacy trials, long-latency side effects are not as well recognized or reported. The high use of ICS/LABA could also be affected by cost because ICS/LABA combinations are less expensive than other combinations. Physician familiarity with ICS/LABA based on ubiquitous use in treating asthma might also be a factor.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2024 recommendations4 highlight indications for ICS use in COPD, as follows: (1) for individuals experiencing ≥2 exacerbations or 1 hospitalization for COPD and with blood eosinophil counts >300 cells/μL as part of triple therapy with ICS/LABA/LAMA and (2) for those with ACO.4 Yet, the most common initial COPD combination drug in the United States is ICS/LABA, which is not the treatment of choice for any GOLD group. This combination ignores the preference for LAMA as the first-line bronchodilator, the demonstrated enhanced bronchodilation of a LAMA/LABA combination, the lack of an ICS indication for those with infrequent exacerbations,4 and the risks of long-term ICS use. These concerns are not well delineated in the GOLD recommendations, which do not mention ICS/LABA. Based on our present results, we believe it is important for clinicians to verify a clinical need for ICS to treat COPD by assessing exacerbation frequency, total eosinophil count, and a failure of LABA/LAMA therapy to provide adequate exacerbation prevention before initiating ICS therapy. Whereas the long-term side effects of ICS therapy are similar for individuals with a concomitant diagnosis of asthma, the risk-benefit ratio of ICS use, the mainstay of asthma treatment, might be very different for those with ACO.
Strengths and LimitationsThis study is based on a very large cohort using EHR data that was opportunistically available for analysis from DARTNet’s Practice Performance Registry. The clinical organizations in the registry might not be representative of all US health care, and thus, the included patients might not be representative of all US individuals. However, the DARTNet registry contains EHR data from several thousand clinical organizations, with sites in all 50 states, ranging from solo clinicians to integrated delivery systems consisting of dozens of hospitals and thousands of clinicians. In addition, the use of genetic matching establishes similar population characteristics between the comparison groups. Thus, the findings are not likely to be affected by using an alternative large EHR data set. Available EHR data contained information on intended therapy and reconciled therapy but does not guarantee that therapies were actually used by individuals. Likewise, evidence of intended treatment extending across multiple years with multiple prescriptions makes it more likely that therapy was intended and likely used. Total use and exact ICS product dispensed are not well delineated in EHR data, and thus we did not attempt to study the effect of different ICS dosages or different types of steroids within an ICS. Nonetheless, the stepwise risk for most outcomes between the short-term, intermediate-term, and long-term ICS groups supports the construct that the findings are based on length of ICS use. We did not use mortality data for these analyses because all data for each individual were truncated at the time of the last live encounter, thus assuring they had the exposure during the period analyzed. We also did not attempt to consider the severity of an individual’s COPD in this analysis; GOLD4 considers severity based on pulmonary function testing, but testing results as discrete data are rarely available in EHRs. Whereas type 2 diabetes and osteoporosis are correlated with COPD,37-42 we could find no literature reporting an increase or decrease in our outcomes of interest associated with COPD severity, when age and therapies used are taken into account. Therefore, lack of pulmonary function–based severity data should not affect the reliability of our findings.
CONCLUSIONSThe long-term effect of ICS use in individuals with COPD is substantial, with greater rates of a composite and individual outcomes of type 2 diabetes, cataracts, pneumonia, osteoporosis, and nontraumatic fracture, as well as recurrent pneumonia and recurrent fracture. The clinical use of and indications for ICS therapy in COPD should be carefully considered for each individual before initiation of long-term ICS therapy.
AcknowledgmentsThe authors gratefully acknowledge Ms Elizabeth Staton’s help in reviewing, editing, and keeping the manuscript concise.
FootnotesConflicts of interest: W.D.P. reports research grant support from Boehringer Ingelheim (outside of this project), AstraZeneca, the Patient-Centered Outcomes Research Institute (PCORI), the National Institutes of Health (NIH), the Office of the National Coordinator for Health Information Technology, the US Centers for Disease Control and Prevention (CDC), and Tabula Rasa Healthcare. He is on the advisory board and is an executive committee member (unpaid) for the COPD Foundation 360 Network; he owns stock through a trust in Johnson & Johnson, Eli Lilly, Novo Nordisk, Pfizer, Novartis, Moderna, and Amgen. E.C. reports research grant support from PCORI, NIH, the Health Resources & Services Administration, the Substance Abuse and Mental Health Services Administration, Otsuka Pharmaceuticals, Merck, Eli Lilly, the AAFP Foundation, Takeda, and the United Health Foundation. She owns stock in Eli Lilly and Pfizer. G.G.-V. reports no conflict of interest. A.S. worked for Boehringer Ingelheim during the time of this research. B.P.Y. reports receiving honoraria from Astra-Zeneca, GlaxoSmithKline, Merck, Teva Pharmaceuticals, and Boehringer Ingelheim for consulting and working on advisory boards, travel support for presentations from AstraZeneca and GlaxoSmithKline, and research funding from AstraZeneca, GlaxoSmithKline, Teva Pharmaceuticals, Boehringer Ingelheim, the National Heart, Lung, and Blood Institute, and PCORI.
Read or post commentaries in response to this article.
Funding support: This work was funded by a research grant from Boehringer Ingelheim Pharmaceuticals, Inc (BIPI). The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). This was a collaborative research study in which BIPI was involved in the design, analysis, or interpretation of the results but was not the regulatory sponsor. The authors received no direct compensation related to the development of the manuscript; BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BIPI substances, as well as intellectual property considerations.
Received for publication January 18, 2024.Revision received December 2, 2024.Accepted for publication December 4, 2024.© 2025 Annals of Family Medicine, Inc.
Comments (0)