ORIGINAL PAPERS / ARTICLES ORIGINAUX
EFFECTS OF LEAD POISONING ON THE DEVELOPMENT OF CHILDREN AGED 1–5 YEARS IN YAOUNDE
Effets du saturnisme sur le développement des enfants âgés de 1 à 5 ans à Yaoundé
ABSTRACT
Background: Lead poisoning affects the central nervous system of children, leading to developmental delay. Herein, we aimed to determine the negative effects of lead poisoning on the development of children aged 1–5 years in Yaoundé.
Methods: Using the Denver II developmental screening test and a pre-tested questionnaire, we assessed the psychomotor development of our participants and collected relevant data. We used Epi info version 3.5.4 and WHO Anthroplus for all data analyses. P <0.05 was statistically significant.
Results: Of the 70 participants included in this study, 22 (31.43%) had blood lead levels of ≥10 μg/dL. Nine (12.86%) of them had developmental delay. We found a significant association between developmental delay and prematurity, low birth weight, artificial milk consumption, postnatal resuscitation, antenatal consultations, prenatal HIV testing, schooling, and high blood lead levels (≥10 μg/dL). However, none of these factors was found to be independently associated with developmental delay.
Conclusion: A blood lead level of ≥10 μg/dL was found to be a risk factor for developmental delay in children aged 1–5 years in Yaoundé. However, developmental delay was found to be a multifactorial outcome. Further prospective studies should be conducted to further investigate our findings.
Keywords: lead, children, psychomotor development, environmental exposure
INTRODUCTION
Lead is the most important toxic heavy element in the environment[1]. Despite its toxicity, this metal has distinctive properties that make its widespread use almost inevitable. Its softness, malleability, ductility, poor conductibility, non-biodegradability, and resistance to corrosion make this heavy metal almost indispensable, and its continuous use causes it to accumulate in the environment, from where human beings are exposed to lead poisoning[2].
Environmental sources of lead exposure include pipe borne water (if the pipes are leaded), aluminum cookware, paints on the walls of houses, paints at playgrounds and parks, lead glazed ceramics[3–6]. Lead poisoning causes hematological, gastrointestinal, and neurological dysfunction in adults and children, and long-term exposure to this heavy metal can cause chronic nephropathy, hypertension, and reproductive impairment[7]. Children are vulnerable to lead toxicity for a number of reasons. Firstly, they can absorb up to 50% of the lead they ingest, unlike the 15–20% absorbed by adults[8]. Secondly, they have a rapid rate of growth and development, which means the lead they take in is quickly assimilated into their organs and systems, disseminating this heavy metal and increasing the scope of its toxicity[9]. Lead poisoning commonly affects the central nervous systems of developing children, especially those aged five years and below. The involvement of the central nervous system negatively affects children’s development[10]. Although the centers for disease control and prevention (CDC) has set the threshold for ‘safe’ blood lead levels (BLLs) at below 10 μg/dL[11], recent studies have demonstrated that BLLs below this cut off value could still negatively affect the central nervous system of children[12]. Furthermore, according to the findings of a study conducted in the USA, blood lead levels as low as 5 μg/dl are still associated with poor cognitive development in children[13]. Although lead poisoning has been associated with poor cognitive development in children[14], not many studies have been conducted in sub-Saharan Africa on this subject. However, in a previous study, the BLLs of 147 children aged 6–72 months in Yaoundé were measured[15]. Since the BLLs of these children were already known, it was possible to investigate the negative effects of this heavy metal on the psychomotor development of children from this cohort. Therefore, in this follow-up study, we aimed to estimate the prevalence of developmental delay, list the domains of developmental delay encountered, and investigate the effects of blood lead levels on the development of children aged 1–5 years in Yaoundé.
MATERIALS AND METHODS
Study design and participants
We conducted a cross-sectional study from February to May of the year 2016 as a follow-up study of a previous study in which the blood lead levels of children aged 6–72 months in Yaoundé were measured [15]. A total of 111 out of 147 children in this study were aged 1–5 years, and the guardians of 72 of them consented to their participation in our study. We obtained complete data in 70 out of the 72 consenting participants. After obtaining their blood lead levels from the principal investigator of the abovementioned study, we went on to assess the psychomotor development of these children using the Denver II developmental screening test[16]. The members of our team were adequately trained on the use of this tool and their knowledge of the tool tested thereafter.
Each child’s psychomotor development was assessed in a quiet room in the child’s home, alone with the investigator and away from possible distractions. The final score for each aspect of psychomotor development (gross motor, fine motor-adaptive, language, and personal-social), alongside the final score of global psychomotor development, was recorded on the questionnaire. These scores were used to compute the developmental quotient using Epi info version 3.5.4. Using the ICD-10 classification of mental and behavioral disorders[17], we determined whether or not the child had a developmental delay, and also the degree of the developmental delay. We classified developmental delay into personal-social, gross motor, fine motor, and global developmental delay. The latter is defined as delay in at least two developmental domains[18].
After assessments of psychomotor development, each child’s nutritional status was determined by measuring parameters such as the weight, height, and mid upper arm circumference using a well-graduated analog precision scale balance (with a 0.5-kg degree of accuracy), stadiometer (with a 0.1-cm degree of accuracy), and measuring tape (with a 0.1-cm degree of accuracy), respectively. With these parameters, we determined each child’s nutritional status using WHO standard growth curves[19] and confirmed it using WHO Anthroplus. The questionnaire was also used to collect information on the child’s sociodemographic status, perinatal history, family history, medical history, present living conditions, level of education (if any), and feeding history. We also collected information on the mother’s obstetric history.
The parents and guardians of all our study participants gave their written informed consent for participation. Ethical clearance was obtained from the National Ethical Committee for Research in Human Health of the Faculty of Medicine and Biomedical Sciences of the University of Yaoundé I.
Data management
The collected data were entered into Epi info version 3.5.4 for analysis. Continuous variables were presented either as mean values and standard deviations (normally distributed data) or median values and interquartile ranges (skewed data). Children’s nutritional statuses were confirmed using WHO Anthroplus. Categorical variables were presented as frequencies and percentages. Mann–Whitney U test (for skewed data) and Student’s t-test (for normally distributed data) were used to perform comparisons between continuous variables, and the chi-square and Fisher exact tests to perform comparisons between categorical variables. All the factors that had p-values of ≤0.2 and odds ratios that were not undefined in the bivariate analyses were entered into a multivariate logistic regression analysis to identify factors that were independently associated with developmental delay. We used odds ratios and their 95% confidence intervals as measures of association between exposure variables and the outcome variable (developmental delay). P-values less than 0.05 were considered statistically significant for all analyses.
RESULTS
Socio-demographic characteristics of the population
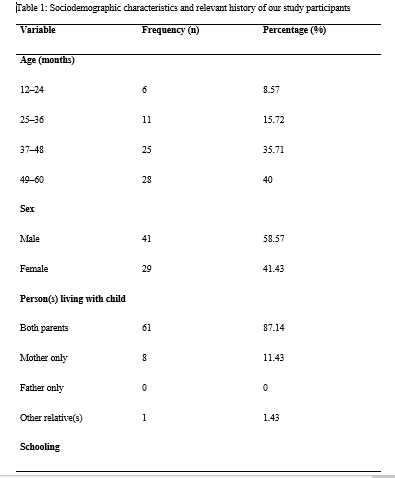
During our study period, we recruited 70 children aged from 12–60 months in Yaoundé. The median age of our study participants [interquartile range, IQR] was 44.5 [37–58] months. The 49–60-month age group was the most represented. There were 41(58.57%) males and 29 (41.23%) females. The majority of our study participants (61, 87.14%) lived with their two parents and 38 (54.29%) of them were schooling. The sociodemographic characteristics and relevant history of our study participants is presented in Table 1.
Maternal obstetric history
Fifty-two (74.29 %) of the 70 mothers of our study participants reported going for antenatal consultations while 18 (25.71 %) reported not going for them. Toxoplasmosis serology was done in 30 (42.86%) cases (and reported to be negative in all of them). Fifty-seven (81.43%) of the mothers declared testing for HIV during pregnancy, and all of them said the results were negative. Thirty-one (44.29%) mothers admitted to have done the syphilis serology during pregnancy and the results were negative. The mothers of 27 (38.57%) children did an antenatal rubella serology which were all reported to be negative.
Six (8.57%) mothers consumed alcohol during pregnancy. The details of the participants’ maternal obstetric history are summarized in Table 1.
Children’s perinatal history
Regarding the place of birth, 38 (54.29%) participants were reported to have been born at health centers, 27 (38.57%) were born in hospitals, while 5 (7.14%) were born at home and taken to a healthcare facility later. Seven (10%) participants were reported to have been born prematurely. Two (2.86%) participants were reportedly resuscitated after delivery (which is indicative of neonatal asphyxia), while 68 (97.14%) did not require resuscitation after delivery.
Only one (1.43%) participant was reported to have suffered from neonatal jaundice, while no study participant had any history of neonatal infections or neonatal convulsions.
Of the 70 participants included in this study, 5 (7.14%) weighed less than 2500 grams at birth, while 65 (92.86%) weighed at least 2500 grams at birth. The details of the perinatal history of the participants are presented in Table 1.
Children’s medical history, feeding history, and nutritional status
Regarding the medical history of the participants, none of them had a history of meningitis, head trauma, sleep disorders, or epilepsy. Of the 70 children, 47 (67.14%) were reportedly exclusively breastfed for the first six months of their lives, while 23 (32.86%) participants were reported to have been on mixed feeding during the first six months of their lives. No child was reported to have been exclusively bottle fed. At the time of data collection, 40 of the 70 children (58.57%) were taking artificial milk.
Of the 70 children, 32 (45.71%) were weaned between the ages of 5 and 14 months, while 30(42.86%) children were weaned between the ages of 15 and 20 months, and 8 (11.43%) children were weaned after the age of 20 months. The age of ablactating, therefore, ranged from 5 months to 26 months, with a mean age of 15.17±4.58 months.
Twelve (17.14%) of the 70 children were malnourished. The feeding history and nutritional status of the study participants are presented in Table 1.
Children’s current living conditions and blood lead levels
Thirty-eight (54.29%) of our seventy study participants were reported to be schooling already, while 32 of them (45.71%) were not schooling at the time of data collection.
As concerns the blood lead levels of the study participants, they ranged from 3.2 μg/dL to 17.6 μg/dL, with a mean value of 8.65±3.75 μg/dL. Twenty-two (31.43%) participants had blood lead levels of ≥10 μg/dL, while 48 (68.57%) had levels of <10 μg/dL. Seven of the children had blood lead levels <5 μg/dL while 63 of them had blood lead levels of ≥5 μg/dL. The living conditions and blood lead levels of the study participants are presented in Table 2.
Assessment of the children’s psychomotor development
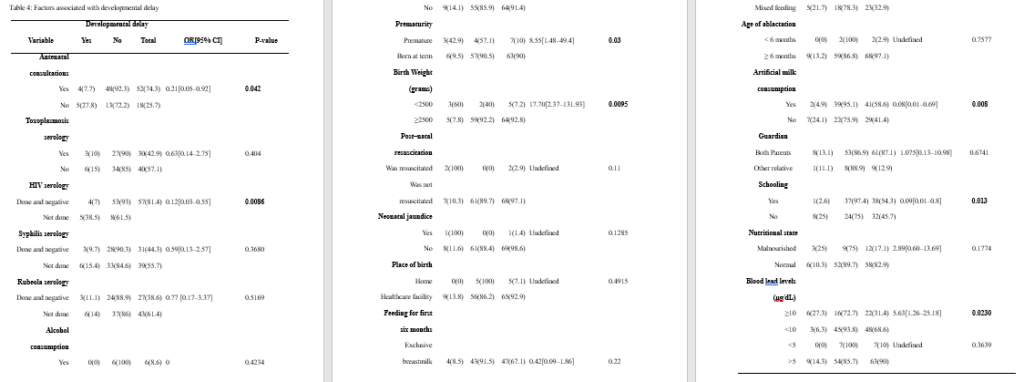
Regarding the psychomotor development of the participants, 61 (87.14%) of them were found to be developing normally for their age, while 9 (12.86%) of them had at least one form of developmental delay. Of the nine children with developmental delay, four had only personal-social delay, three had global developmental delay (personal-social and fine motor delay), while one each had isolated language and fine motor delay. The details of the psychomotor development of our study participants and the types of developmental delay encountered are presented in Table 3.
Associations between various exposure variables and developmental delay
We found significant associations between prematurity, schooling, maternal HIV serology results, low birth weight, mothers going for antenatal consultations during pregnancy, artificial milk consumption, and high blood lead levels (at a threshold of 10 μg/dL). The results of the univariate analysis performed with developmental delay as the outcome variable are presented in Table 4.
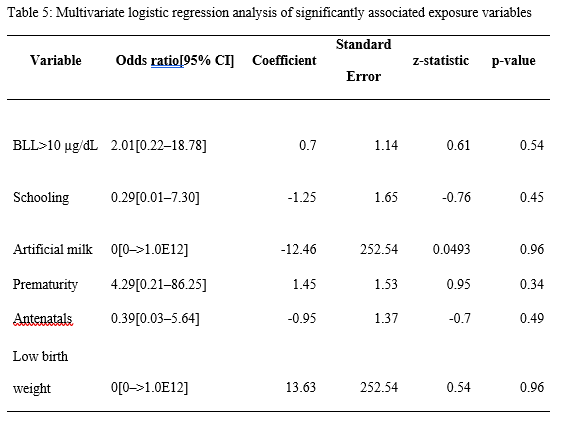
Multivariate logistic regression analysis
After identifying all the factors that were significantly associated with developmental delay in our study participants, we performed a multivariate logistic regression analysis to further investigate if any of these factors were independently associated with the outcome variable. However, we found that none of them was independently associated with developmental delay in our study population. The results of our multivariate logistic regression analysis are presented in Table 5.
DISCUSSION
We conducted this study to estimate the prevalence of developmental delay and investigate the effect of lead poisoning on the development of children aged 1–5 years in Yaoundé. The median age of the 70 children we included was 44.5 [37–58] months, which is higher than the mean age of 26.5±13.4 months reported by Bishworkama et al. in Nepal in 2022. This discrepancy could be explained by the fact that they had a larger study population (165 children aged 7–57 months) than ours. Murphy et al. reported a median age of 4 [3–4] years in their study conducted in Malawi, despite studying children aged 2–10 years. This finding agrees more with those of our study, which underlines the fact that developmental delay is more common among pre-school children[20], especially in low- and middle-income countries, where children are more exposed to factors that contribute to developmental delay[21]. The prevalence of developmental delay per our findings was 12.86%, with personal-social delay being the most common type of delay. This is in line with the 10–15% reported by Vitrakas et al.[22]. However, the occurence of developmental delay decreases with increasing age, as reported by Zhang et al., who found that, the prevalence among children aged 6–11 months was almost double that of children aged 30–35 months in a study conducted in rural China[21]. Furthermore, according to another study, not all children with developmental delay will have a developmental disability in the long run, as most of them tend to catch up later in life[23]. Thus, the effective early identification of developmental delays and timely early intervention can positively alter a child’s long-term trajectory[24]. The blood lead levels of our study participants ranged from 3.2 μg/dL to 17.6 μg/dL, with a mean value of 8.65 ± 3.75 μg/dL, and 31.43% of participants having blood lead levels of ≥10 μg/dL. This proportion is lower than that reported by Irawati et al. in Indonesia (69.5%), mainly because Irawati et al. conducted their study among children who were living at used lead acid battery recycling sites. This means the children in their study had significantly higher exposures to this heavy metal compared to those in our study, who had no evidence of industrial lead exposure. Contrary to the findings of Irawati et al., Kashala-Abotnes et al. reported a mean blood lead level of 6.9 ± 4.8 μg/dL in children aged 12 to 24 months living in Kingshasa with no industrial lead exposure[25], which agrees more with our findings. The prevalence of developmental delay among children with lead poisoning was 27.3%, and this outcome was significantly more common among children with lead poisoning compared to children with BLL > 10 μg/dL[Office1] . This is in line with the findings of previous studies that identified lead as the most common environmental neurotoxin[6].
We found that antenatal visits during pregnancy were associated with developmental delay. This agrees with the findings of Ergaz and Ornoy, who reported that maternal factors including weight, diet, and morbidities can affect neonatal adaptation and later development[26]. The mother’s willingness to pay for and receive adequate antenatal care in this part of the world is a sign that the parents of the unborn baby are not only enlightened but also financially buoyant, as a study conducted in Nigeria identified poverty as the main reason why pregnant women do not go for antenatal visits[27]. Also, it is often during antenatal visits that women get to do their HIV serological tests during pregnancy, which was another factor associated with developmental delay in our study. We also identified prematurity and low birth weight as factors significantly associated with developmental delay, which is in line with the findings of a previous study conducted in Canada, according to which preterm infants were significantly more predisposed to developmental delay than infants born at term[28]. Cheong et al. also reported similar findings in Australia[29]. The inferior neurodevelopment of preterm infants is multifactorial and is likely a consequence of an immature brain, perinatal risk factors, and environmental exposures, with the immaturity of their brains being the major contributing factor in early childhood[30]. However, the influence of perinatal factors wanes over time as these children tend to catch up on their psychomotor development[31]. Postnatal resuscitation was another factor significantly associated with developmental delay, which is in line with the findings of a previous study that reported poorer cognitive outcomes for babies who required postnatal resuscitation[32]. This is probably because perinatal physiological compromise might be sufficient to cause subtle neuronal or synaptic damage, and thereby affect cognition in childhood and potentially in adulthood. Current artificial milk consumption was another factor associated with developmental delay in this study. This could be because artificial milk contains high levels of energy, proteins, micro- and macronutrients, calcium, and insulin-like growth factor-1, which are all required for children’s development and growth[33]. Also, in Sub-Saharan Africa, milk consumption is generally associated with a high socioeconomic status, which means children who are constantly being fed dairy products are less exposed to environmental risk factors for developmental delay. We identified schooling as another factor significantly associated with developmental delay. Although developmental delays have been identified as one of the etiologies of the partial disturbances in performance frequently resulting in failure at school[34], schooling could help a child catch up by creating a platform for interactions with other kids of the same age group. Finally, we identified a high blood lead level as another factor significantly associated with developmental delays in our study population, which is in line with the findings of Delgado et al.[35]. This is not uncommon, as lead has already been identified as the most common environmental neurotoxin[6]. Thus, this heavy metal probably hinders the psychomotor development of infants and young children after finding its way to their central nervous systems. Nevertheless, our multivariate logistic regression analysis did not identify any factor independently associated with developmental delay, which suggests that developmental delay is an adverse outcome with a multifactorial etiology[20,28].
In conclusion, we found that 31.43% of children aged 1–5 years in Yaoundé had blood lead levels of ≥10 μg/dL, and 12.86% of them had at least one form of developmental delay, of which personal-social delay was the most commonly encountered type. The factors associated with developmental delay in our study were antenatal visits, HIV testing during pregnancy, prematurity, low birth weight, postnatal resuscitation, current milk consumption, schooling, and high blood lead levels (≥10 μg/dL). However, no single factor was independently associated with developmental delay in this study, which means this adverse outcome has a multifactorial etiology. Our study had certain limitations. Firstly, our sample size was small. Secondly, some of the data we collected were not documented, and the parents of our participants simply had to give us the required information as they remembered it. The recall bias associated with this method of data collection could have affected the quality of some of our findings. Third, we used the Denver II developmental screening test, which has limited specificity (43%)[36]. Thus, it is possible that some of our participants were diagnosed with developmental delay whereas they were developing normally. With all these limitations, further large-scale prospective studies using better-suited screening tests should be performed to further investigate our findings.
Acknowledgments: The authors thank the parents and guardians of the study participants for their full cooperation.
Funding: The authors did not receive any funding for this study.
Competing interests: The authors have no conflicts of interest to declare.
Authors’ contributions: CA, LN, and JEA conceived and designed the study; CA and JEA wrote the first draft of the manuscript; DTE and CA analyzed the data; LN and DTE critically reviewed the manuscript. All authors approved the final version of the manuscript.
Data availability statement: The data analyzed and presented in this study are available from the corresponding author upon reasonable request.


REFERENCES
WANI AL, ARA A, USMANI JA. Lead toxicity: a review. Interdiscip Toxicol. 2015;8:55-64. LANDRIGAN PJ, TODD AC. Lead poisoning. West J Med. 1994;161:153-9. WEIDENHAMER JD, KOBUNSKI PA, KUEPOUO G, CORBIN RW, GOTTESFELD P. Lead exposure from aluminum cookware in Cameroon. Sci Total Environ. 2014;496:339-47. MATHEE A, RÖLLIN H, LEVIN J, NAIK I. Lead in Paint: Three Decades Later and Still a Hazard for African Children? Environ Health Perspect. 2007;115:321-2. MATHEE A, SINGH E, MOGOTSI M, TIMOTHY G, MADUKA B, OLIVIER J, ING D. Lead-based paint on playground equipment in public children’s parks in Johannesburg, Tshwane and Ekurhuleni. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2009;99:819-21. TERRAZAS-MERAZ MA, HERNÁNDEZ-CADENA L, RUEDA-HERNÁNDEZ GE, ROMANO-RIQUER SP, SHAMAH-LEVY T, VILLALPANDO-HERNÁNDEZ S, SOLIS MMT-R, HERNÁNDEZ-AVILA M. [Use of lead-glazed ceramic as a source of exposure in children of marginalized indigenous zones of Oaxaca, Mexico]. Salud Publica Mex. 2015;57:260-4. LOCKITCH G. Perspectives on lead toxicity. Clin Biochem. 1993;26:371-81. UNEP, UNICEF Childhood lead poisoning: Information for advocacy and action. 1999. LIU J1, LI L, WANG Y, YAN C, LIU X. Impact of low blood lead concentrations on IQ and school performance in Chinese children. PLoS One. 2013;8:e65230. Centers for Disease Control and Prevention (CDC). Very high blood lead levels among adults – United States, 2002-2011. MMWR Morb Mortal Wkly Rep. 2013;62:967-71. MIN MO1, SINGER LT, KIRCHNER HL, MINNES S, SHORT E, HUSSAIN Z, NELSON S. Cognitive development and low-level lead exposure in poly-drug exposed children. Neurotoxicol Teratol. 2009;31:225-31. TON S, PRAPAMONTOL T, SCHIRNDING YE. Environmental lead exposure: a public health problem of global dimensions. Bull World Health Organ. 2000;78:1068-77. AHMADI S, LE BOT B, ZOUMENOU R, DURAND S, FIÉVET N, AYOTTE P, MASSOUGBODJI A, ALAO MJ, COT M, GLORENNEC P, BODEAU-LIVINEC F. Follow-Up of Elevated Blood Lead Levels and Sources in a Cohort of Children in Benin. Int J Environ Res Public Health. 2020;17:8689. MONEBENIMP F, KUEPOUO G, CHELO D, ANATOLE PC, KANY BISSEK ACZ, GOTTESFELD P. Blood Lead Levels among Children in Yaoundé Cameroon. Front Public Health. 2017;5:163. FRANKENBURG WK, DODDS J, ARCHER P, SHAPIRO H, BRESNICK B. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics. 1992;89:91-7. SARTORIUS N. [Classification of mental disorders according to ICD 10]. L’Encephale. 1995;21:9-13. VASUDEVAN P, SURI M. A clinical approach to developmental delay and intellectual disability. Clin Med. 2017;17:558-61. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Oslo Nor 1992 Suppl. 2006;450:76-85. GIL JD, EWERLING F, FERREIRA LZ, BARROS AJ. Early childhood suspected developmental delay in 63 low- and middle-income countries: Large within- and between-country inequalities documented using national health surveys. J Glob Health. 10:010427. ZHANG J, GUO S, LI Y, WEI Q, ZHANG C, WANG X, LUO S, ZHAO C, SCHERPBIA RW. Factors influencing developmental delay among young children in poor rural China: a latent variable approach. BMJ Open. 2018;8:e021628. VITRIKAS K, SAVARD D, BUCAJ M. Developmental Delay: When and How to Screen. Am Fam Physician. 2017;96:36-43. CHOO YY, AGARWAL P, HOW CH, YELESWARAPU SP. Developmental delay: identification and management at primary care level. Singapore Med J. 2019;60:119-23. SCHERZER AL, CHHAGAN M, KAUCHALI S, SUSSER E. Global perspective on early diagnosis and intervention for children with developmental delays and disabilities. Dev Med Child Neurol. 2012;54:1079-84. KASHALA-ABOTNES E, MUMBERE PP, MISHIKA JM, NDJUKENDI AO, MPAKA DB, BUMOKO MMG, KAYEMBE TK, TSHALA-KATUMBAY D, KAZADI TK, OKITUNDU DLE-A. Lead exposure and early child neurodevelopment among children 12-24 months in Kinshasa, the Democratic Republic of Congo. Eur Child Adolesc Psychiatry. 2016;25:1361-7. ERGAZ Z, ORNOY A. Perinatal and early postnatal factors underlying developmental delay and disabilities. Dev Disabil Res Rev. 2011;17:59-70. FAGBAMIGBE AF, IDEMUDIA ES. Barriers to antenatal care use in Nigeria: evidences from non-users and implications for maternal health programming. BMC Pregnancy Childbirth. 2015;15:95. BALLANTYNE M, BENZIES KM, MCDONALD S, MAGILL-EVANS J, TOUGH S. Risk of developmental delay: Comparison of late preterm and full term Canadian infants at age 12 months. Early Hum Dev. 2016;101:27-32. CHEONG JL, DOYLE LW, BURNETT AC, LEE KJ, WALSH JM, POTTER CR, TREYVAUD K, THOMPSON DK, OLSEN JE, ANDERSON PJ, SPITTLE AJ. Association Between Moderate and Late Preterm Birth and Neurodevelopment and Social-Emotional Development at Age 2 Years. JAMA Pediatr. 2017;171:e164805. HEE CHUNG E, CHOU J, BROWN KA. Neurodevelopmental outcomes of preterm infants: a recent literature review. Transl Pediatr. 2020;9:S3-8. DO CHT, KRUSE AY, WILLS B, SABANATHAN S, CLAPHAM H, PEDERSEN FK, PHAM TN, VU PM, BØRRESEN ML. Neurodevelopment at 2 years corrected age among Vietnamese preterm infants. Arch Dis Child. 2020;105:134-40. ODD DE, LEWIS G, WHITELAW A, GUNNELL D. Resuscitation at birth and cognition at 8 years of age: a cohort study. Lancet Lond Engl. 2009;373:1615-22. HERBER C, BOGLER L, SUBRAMANIAN SV, VOLLMER S. Association between milk consumption and child growth for children aged 6-59 months. Sci Rep. 2020;10:6730. WOHLFEIL A. [Developmental delay in students starting school and possibilities for detection in preschool screening by the public health office]. Offentliche Gesundheitswesen. 1991;53:474-81. DELGADO CF, ULLERY MA, JORDAN M, DUCLOS C, RAJAGOPALAN S, SCOTT K. Lead Exposure and Developmental Disabilities in Preschool-Aged Children. J Public Health Manag Pract JPHMP. 2018;24:e10-17. GLASCOE FP, BYRNE KE, ASHFORD LG, JOHNSON KL, CHANG B, STRICKLAND B. Accuracy of the Denver-II in developmental screening. Pediatrics. 1992;89:1221-5.
Comments (0)