Bladder cancer (BC), one of the most common cancers worldwide, is particularly prevalent in developed countries [1, 2]. Its global incidence was estimated to be equal to 3%, in 2020, according to GLOBOCAN [1, 2]. It is a complex disease that involves different risk factors, mainly smoking and occupational carcinogen exposure [3], in addition to genetic susceptibility [4]. In fact, the lifetime absolute risk (AR) of developing BC in 50 years-old white non-Hispanic never-smoker men is 1.9%, whereas the AR is 7.1% for current smokers among 50 years-old white non-Hispanic men [5]. Recent advances in DNA sequencing technologies, in particular next-generation sequencing approaches, have opened new horizons, allowing a better understanding of genetic triggers related to BC [6]. Several genomic alterations were found to be linked to this specific cancer, including gene rearrangements, amplifications, deletions, copy number variations, and point variants, including pathogenic and polymorphic variants that are also known as single nucleotide polymorphisms (SNPs) [7]. SNPs, which account for 90% of the human genome’s variability, are gaining much interest in the oncogenetics field since many of them have been shown to modulate cancer susceptibility by increasing or decreasing the risk of an individual developing cancer [8]. Among genes associated with cancer, we can cite genes regulating environmental carcinogen metabolism, DNA repair, or cell cycle pathways, all of which are involved in the development and/or progression of any type of cancer, including BC [9, 10].
Many studies have examined the association of SNPs with BC in different populations. This extensive literature review aims to list all SNPs that are significantly associated with BC and discuss their involvement in this type of cancer.
MethodsIn order to gather all available information related to the possible association of various SNPs with BC, an extensive literature search was performed in the PubMed database covering the period between January 2000 and October 2020. The keywords “bladder cancer” combined with “genetic predisposition” were used with Boolean operators. Overall, 894 articles were selected. The titles and abstracts of these articles were assessed for eligibility before evaluating their contents. Articles that target hereditary cancer, epigenetic studies, and linkage analysis studies were not included. At the end, 334 articles were selected, reporting 455 SNPs located in 244 genes. Selected genes were then classified based on the same method used by [11]. The article selection process is summarized in Figure 1 as a PRISMA flow diagram.
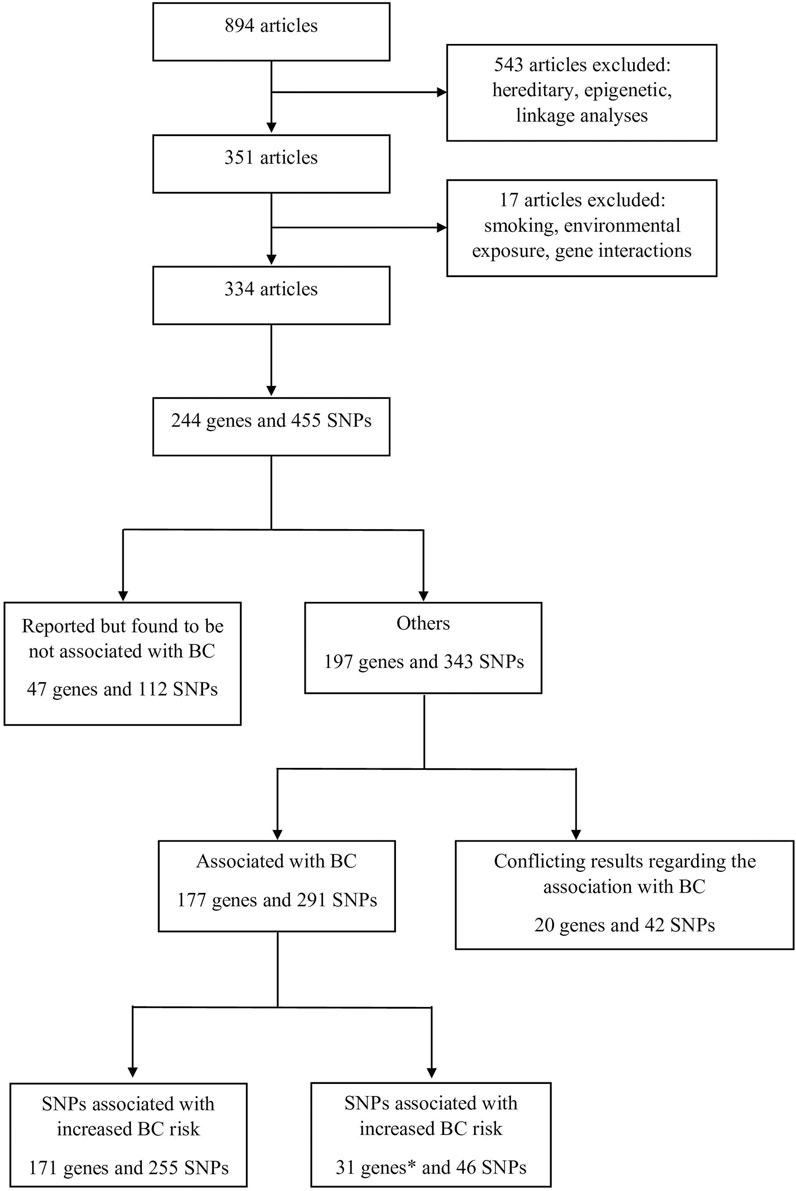
FIGURE 1. Process of the selection of the articles that were included in this study.* Genes with some SNPs that increase BC risk and others that decrease BC risk were counted twice.
The selected 455 SNPs were further investigated. All SNPs that were associated with smoking and environmental exposure but did not present a direct association with BC were excluded from this study. However, SNPs associated with BC independently of interfering factors like smoking status, sex, age, and others were included.
ResultsIn total, 197 genes and 343 SNPs were found to be associated with BC, of which 177 genes and 291 SNPs had congruent results between all available studies, meaning that all studies that assessed a particular SNP had the same results regarding the SNP association with BC. The remaining 20 genes and 42 SNPs were reported with conflicting data, thus representing a huge amount of scattered data that needs a clear classification in order to facilitate their accessibility to researchers. These genes and SNPs were thus thoroughly evaluated and classified into eight different categories according to their function: chemical carcinogenesis, signaling, cell death, DNA repair, cell cycle, cell architecture, metabolic, and other genes that do not correspond to any specific category. Further subclassifications were also enclosed in each category (Table 1).
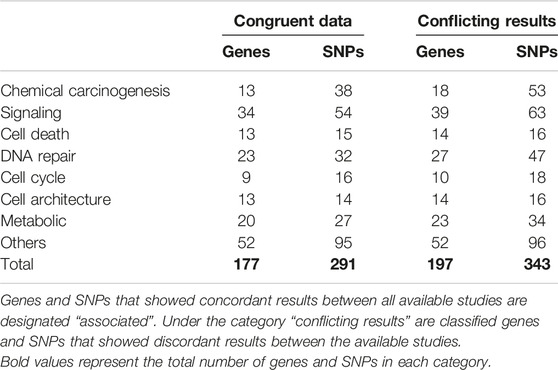
TABLE 1. Summary of genes classification with number of genes in each category.
Chemical CarcinogenesisGenetics and environmental factors impact the quantities of enzymes that participate in the activation and detoxification of chemicals, which can potentially lead to carcinogenesis. Most chemical compounds become carcinogens after being metabolized to chemically reactive electrophiles, which can interact with DNA to generate a carcinogenic response [12]. Genes encoding enzymes modulating chemical carcinogenesis were found to be linked to BC. In this section, genes were divided according to the different known enzyme families. Genes that encode for phase I and phase II enzymes were subcategorized into four categories: Cytochromes, Glutathione S-Transferase, N-acetyltransferase, and UDP-Glucuronosyltransferase (UGT).
Cytochromes are phase I enzymes whose role is to convert xenobiotics into excretable compounds. They are divided into subfamilies and are primarily located in the liver, but can also be found in the gastrointestinal tract, lungs, and kidneys. Mutations in these genes can affect substrate transformation, which may lead, in some cases, to cancer [13]. For instance, rs2472299 in CYP1A is known worldwide as a risk factor for BC [13], while rs4646903 and rs2198843 in the same gene [13, 14] are known to widely increase the risk for the same type of cancer but exclusively in the Asian population, and rs4646421 is specific for BC in Tunisians [15]. Results regarding rs1048943 in CYP1A1 are contradictory, since it increases the risk for BC in Asian and Brazilian populations but was not associated with BC in many other studies [13, 16–19]. Similarly, rs762551in CYP1A2 showed conflicting results [20–22]. Regarding the CYP1B1 gene: rs2855658 increases BC risks in the European population [23], and rs10012, rs1056827, and rs150799650 showed an increased risk in Indo-European cohorts [24], while rs1056827 was associated with a decreased risk in the Spanish population [25]. Contradictory results were seen for rs1056836, which presents a notable increased risk for BC in the Spanish population [13, 19, 25, 26]. On the other hand, rs4244285 and rs4986893 in CYP2C19 showed a protective effect against BC in the Chinese population [27]. CYP2E1 was associated with an increased risk for BC in the Lebanese population [28]. However, rs2031920 in the same gene was widely associated with a decreased risk in Asians [29] with controversial results in Caucasians [29, 30].
Glutathione S-Transferase constitutes a superfamily of phase II multipurpose enzymes that contribute to metabolic detoxification processes that protect macromolecules from environmental carcinogens, reactive oxygen species, and chemotherapeutic agents. The glutathione S-transferase family includes the following enzymes: GSTA, GSTM, GSTP, GSTT, and GSTO. Since these molecules contribute to the metabolism of potential carcinogens, any polymorphism affecting their expression or function may lead to cancer [31]. Most articles show that in most populations, the GSTM1 null genotype [32–47] and rs1695 in GSTP1 [16, 19, 33, 40, 42, 48–55] increase the risk for BC. However, a Chinese study showed that the GSTT1 null genotype was associated with a decreased risk for BC [36]. Rs156697 in GSTO2 also shows a positive association with BC in the Serbian population [56]. The polymorphisms in GSTT1 were mostly associated with an increased risk for BC [16, 18, 32–34, 40, 42, 44, 47, 48, 57–63]. Rs3957356, rs3957357, and rs4715332 in GSTA1 were associated with an increased risk for BC in both Balkan-Ben and Balkan-non Ben [63].
N-acetyltransferase (NAT) enzymes, also known as NAT1 and NAT2, are responsible for acetylating aromatic and heterocyclic amines in the liver, the gastrointestinal tract, and the urinary bladder. Genetic polymorphisms in these enzymes can alter the processing rate of various carcinogenic compounds, thus increasing the risk for cancer. Hence, slow NAT2 acetylation is associated with increased risk for BC [64]. Many SNPs in NAT1 are associated with a significantly increased risk for BC in the Lebanese population, including rs15561, rs4986782, and rs1057126 [28, 65, 66]. However, the latter did not show any association with BC in two other meta-analyses [4, 67]. Rs9650592 in NAT1 was positively associated with BC in the European population [23]. A study conducted on a French Caucasian population demonstrated an increased risk for BC in the presence of rs1208, rs1801280, or rs1041983 in NAT2 [19]. Rs1041983 presents the same effect in the American population [40]. Rs1799929, rs1799930, and rs1799931 were shown to be associated with BC in French and Bangladeshi populations [19, 68]. However, a Chinese meta-analysis contradicted the French results and found no association between these SNPs and BC [69]. Finally, rs1495741 and rs4646249 increase the risk of BC [11, 37, 70] in the European population [23].
The UDP-Glucuronosyltransferase (UGT) family of enzymes are phase II enzymes that are involved in the glucuronidation of aromatic amines and other carcinogens. They are primarily located in the liver but also in the gastrointestinal tract, lungs, and kidneys. The UGT family is composed of UGT1A, UGT2A, and UGT2B. They present different subtypes that are all, except for UGT2B17, expressed in normal bladder tissue [71]. Conflicting data was documented regarding the association of rs11892031 in UGT1A10/UGT1A8 with BC [23, 39, 70, 72–75]. Rs17863783 in UGT1A6 decreases the risk of developing BC [70, 76]. Rs1104892, rs1105880, rs1113193, rs1604144, rs17854828, rs17864684, rs17868322, rs2602374, rs2741042, rs2741044, rs2741045, and rs4148326 in UGT1A8 are associated with an increased risk in the American population, while rs1113193, rs1604144, rs17854828, rs17864684, rs4148328, rs4233633, and rs7571337 are associated with a decreased risk in the same population [77]. Rs2736520 in UGT2B4 increases the risk for BC, while Rs3822179 decreases the risk in the American population [77].
CDC-like kinase 3 (CLK3) is a dual specificity kinase that belongs to the serine/threonine type kinase family. Its role in human cancer is still undetermined [78]. However, a Japanese study has established that rs11543198 is associated with an increased risk for BC in the Japanese population [79].
SignalingSignaling and immune-related genes were found to be associated with BC in the literature. These genes were divided into four categories, including cytokines, toll-like receptors, transcriptional factors, and other genes.
For an effective immune response against cancer cells, a sequence of steps must take place in fighting tumor cells. Each step of this sequence requires the presence of several stimulatory and inhibitory factors [80]. Among these actors, Cluster of Differentiation (CD) 274 [also known as Programmed Death Ligand 1 (PD-L1)], has an inhibitory function toward immune response activation. Rs4143815 in this gene increases the risk for BC in the Chinese population [81]. Cytotoxic T-lymphocyte-associated protein 4 (CTLA4) can also inhibit T cell-mediated immune responses, and rs231775 and rs3087243 in the corresponding gene have been shown to increase the risk for BC in the Indian population [82]. Intercellular Adhesion Molecule 1 (ICAM1) is responsible for T cell tumor infiltration, and therefore a variation in this gene can increase cancer susceptibility, as shown for rs5498 in the Taiwanese population [83]. Furthermore, chemokines help regulate the immune response and impact cancer progression [84]. Among the genes encoding chemokines, C-C chemokine receptor type 2 (CCR2) rs1799864 [85–87] and CCR5 rs333 [85] were associated with an increased risk for BC in the Turkish population, rs1801157 in C-X-C motif chemokine 12 (CXCL12) also has a positive association with BC in Indian [88] and Turkish [85] populations; and rs1126579 in CXC chemokine receptors 2 (CXCR2) increases cancer’s susceptibility in the Indian population [88] while it shows a protective effect towards BC in the American population [89]. Finally, rs187115 in CD44 was associated with an increased risk for BC in the Taiwanese population [90]. Tumor-secreted T-helper 17 (Th17) cell-associated interleukins (IL) mobilize immune suppressive cells and promote tumor growth [91]. Rs2275913 in IL-17A and rs187238 in IL-18 increase the risk for BC in the Polish population, with the highest association for IL-18 [92]. Rs1946518 in IL-18 also increases the risk of BC in the Indian population [93]. Rs2227485 in IL-22 is also a risk factor for BC in the Chinese population [94]. Other interleukins that play a role in macrophages and neutrophils’ immunosuppressive roles include IL-10, IL-12, and IL-23 [95]. Rs1800896, rs1800871, and rs1800872 in IL-10 [96] are associated with an increased risk for BC in Chinese [97] and Indian [98] populations; rs10889677 in IL-23R also increases BC risk in Chinese [99] and Polish [92] populations; and IL-12 decreases the risk in the Indian population [93]. Other interleukins were also found to play a role in BC risk; for instance, rs2069762 in the IL-2 gene increases the risk for BC in the Chinese population. Rs4073 in IL-8/CXCL8 gives high BC susceptibility in the Indian population [100] and rs1800890 in IL-19 in the Spanish population [101]. Last but not least, rs153109 and rs17855750 in IL-27 predispose to BC in Spanish and Chinese individuals, respectively [102, 103]. Finally, rs1799964 in TNF-α [104] and rs1800470 in TGFB1 [105] are positively associated with BC in the Indian population.
Toll-like receptors (TLR) play a key role in the initiation of the innate immune response. They are expressed on both immune and tumor cells and regulate the immune responses in tumor progression and as therapeutic targets for cancer [106]. TLR2 mutations (−196 to −174del) increase the risk of BC in India [107]. Rs11536889 in TLR4 [108–110] and rs72552316 in TLR7 [111] constitute separate BC risk factors.
Transcription factors might be linked to BC [112]. For instance, rs2228570 in VDR (Vitamin D receptor) was found to increase the risk of developing BC in India [113] and Tunisia [114]. TP63 can act as a tumor suppressor gene or as an oncogene depending on the cellular setting and pathways where it is implicated, and it can thus regulate the transcription of different genes [115]. Several SNPs have been shown to increase the risk of BC, among them rs4687100 [116] and rs710521 [39, 70, 116]. Of note, the latter has also been proven to present a protective effect in the Indian population [117], but no association with BC was identified in a Chinese study [118]. Finally, conflicting data were also obtained for rs35592567 since it was shown to have a positive association in one meta-analysis [11] and a negative association in another [119].
Vascular endothelial growth factor (VEGF) regulates angiogenesis and is upregulated and overexpressed in BC [120]. Rs699947 and Rs35569394 in VEGF increase and decrease, respectively, the BC risk in the Indian population [121]. Moreover, rs833052, rs25648 [122], rs3025039 [122, 123], and rs699947 [124] in VEGFA showed a positive association with BC, while the latter also presented a protective effect in the Tunisian population [125]. Rs3775194, rs1485762, rs6828869, and rs17697515 in VEGFC, and rs4557213 in VEGFR increase the risk for BC in the American Caucasian population [126] while rs1485766 in VEGFC increases the risk in the Taiwanese population [127]. Human Leukocyte Antigen G (HLA-G) expression in tumors promotes the immune suppressive microenvironment, which results in poor treatment response and prognosis [128]. Rs1063320, rs1610696, rs1704, rs1707, rs1710, rs17179101, and rs17179108 in HLA-G are associated with increased cancer susceptibility in the Brazilian population [129].
Other SNPs that were also studied in various genes include: rs6593205 and rs7799627 in Epidermal Growth Factor Receptor (EGFR), which decrease the risk for BC in the American Caucasian population, while rs11238349 increases the risk in the same population [126] and rs1050171 increases the risk in the Chinese population [130]. Rs696 in NFKBIA and Rs11188660 in BLNK (B cell linker) increase the risk in the Spanish population [101]. Rs28362491 in NFKB1 [131–133] and rs7944701 in MAML2 [134] increase the risk in the Chinese population. A more extensive list is available in Supplementary Table S1 under the section titled “signaling.”
Cell DeathBy evading death, immortal cells can develop into tumors. Mutations in certain pathways promoting cell death can thus lead to tumor formation. Cell death pathways involve multiple genes, including cell-death receptors such as Tumor Necrosis Factor Receptor 1 (TNFR1), FAS, and TNF-related apoptosis-inducing ligand (TRAIL) receptors and effectors such as caspases [135]. Genes and SNPs related to those pathways were reported in BC. Rs2234767 in FAS, Rs763110 in FASLG [136], and Rs1131580 in TNFSF10 [137] increase the risk for BC in the Turkish population. In DR4 (TRAILR1 or TNFRSF10A), rs6557634 increases the risk for BC the Indian population [138] and rs13278062 increases the risk in the Chinese population [139] while rs20575 was found to present a protective effect in the Caucasian population [140]. Moreover, rs2647396 in BCL10 [101], rs10999426 in PRF1 [101], and rs42490 in RIPK2 [141] each individually increase the risk for BC in the Spanish population, while rs401681 in CLPTM1L increases the risk widely in all studied populations [39, 73, 74]. Rs4647603 in CASP3 and rs3181320 and rs507879 in CASP5 increase the risk for BC in Indians [138] while rs3834129 in CASP8 [142] and rs4645978 in CASP9 [143] decrease the risk for BC in the Chinese and Indian populations, respectively. Finally, rs1045411 in HMGB1 presents a protective effect in the Chinese population [144].
DNA RepairThe DNA repair genes associated with BC are classified according to the repair mechanism: base excision repair (BER), nucleotide excision repair (NER), homologous recombination (HR), and poly-ADP-ribosylation.
BER is a mechanism of DNA reparation that is not restricted to the repair of single-strand breaks but also of damages resulting from defects in alkylation, oxidation, deamination, and depurination. Given that this mechanism repairs thousands of errors per cell and per day, it plays a major role in cancer prevention by ensuring genome integrity and stability. Therefore, BER guarantees the integrality of apoptosis pathways and prevents mutation accumulation that may initiate tumors [145]. In X-ray repair cross-complementing protein 1 (XRCC1), rs1799782 and rs25489 are widely associated with an increased risk for BC, especially in Asian and Indian populations [146–152]. Rs915927 in the same gene increases tumor susceptibility in the Italian population [153] while rs25487 gave contradictory results in different studies [147–149, 154–159]. Rs3218373, rs3218536, and rs6464268 in XRCC2 present a protective effect towards BC in the Italian population [160]. Rs1805377 in XRCC4 increases the risk of BC in Spanish [160] and Indian individuals [161]. Rs6869366 increases the risk of BC in Taiwanese people [162] but decreases the risk in the Indian population [161]. Rs828907 in XRCC5 increases BC risk in the Taiwanese population [163]. Rs3213245 and rs7003908 in XRCC7 (PRKDC) are positively associated with BC, respectively, in the Chinese Han [164] and in the Indians [165]. Other SNPs in BC-associated BER genes include rs1760944 in APEX1, which decreases the risk for BC in the Chinese population [146] while rs1130409 in APE1 showed conflicting data [147, 166, 167]. Rs3136717 in POLB [168] and rs1052133 in hOGG1 [147, 165, 169–171] are associated with a high risk for BC, while rs125701 in hOGG1 decreases the risk for BC in the Spanish population [168]. Finally, rs2029167 in SMUG1 and rs3219487 in MUTYH increase the risk for BC in the American population [172] and rs11039130 in DDB2 has the same effect in the Caucasian population [173].
NER is also an important DNA repair pathway consisting of two distinct sub-pathways. The global-genome NER process fixes helix damages over the entire genome, whereas the transcription-coupled NER mechanism acts during transcription to resolve RNA polymerase blocking lesions [174]. Mutations in genes implicated in NER can result in a predisposition to cancer since mutations and chromosomal abnormalities can either activate oncogenes or inactivate tumor suppressor genes [175]. Rs3212961 in ERCC1 increases BC risk in the Spanish population [176] whereas rs967591, rs735482, and rs2336219 have a protective effect towards BC in the Italian population [153]. Rs13181 [15, 157, 177–182], rs1799793 [166, 170, 177, 178, 183–186] and rs238406 [176, 178, 179] in ERCC2 (XPD) were individually widely associated with an increased BC risk. Rs1047769 [176] and Rs17655 [187] in ERCC5 increase the risk of BC, respectively, in Spanish and Chinese people. Rs2228526 and rs2228528 in ERCC6 increase BC susceptibility in Belarussians [181] with the latter also having the same effect in the Taiwanese population [188]. Rs4150667 in GTF2H1 is associated with an increased risk for BC in the Caucasian race [173], rs1805335 in RAD23B increases the risk in the Spanish population [176] and rs2228000 [189–193] and rs2228001 [155, 187, 193–195] in XPC are positively associated with BC in all study populations.
HR is another mechanism that helps maintain the genome’s integrity by repairing double-strand breaks. Therefore, HR deficiencies that result from gene mutations make individuals more susceptible to cancer [196]. Rs861539 in XRCC3 is associated with an increased risk for BC in different populations [160, 197–199]. Rs1799794 and rs861530 in the same gene also increase BC risk in the Chinese population [200]. Rs11571833 in BRCA2 increases BC risk in those of European descents [201]. Rs1805794 in NBN is associated with a high risk for BC [202] while rs8032440 in FANCI and rs3739177 in PNKP make the American population more susceptible to BC [172].
Poly-ADP-ribosylation is a post-translational modification catalyzed by poly(ADP-ribosyl)ation polymerases (PARPs) as a response to DNA damage [203]. Rs1136410 in PARP1 is associated with a high BC risk in the Spanish population [168], whereas rs3219123 and rs12568297 in PARP1, rs1713413 in PARP2, and rs2862907 in PARP4 increase separately the risk for BC in the American population [172].
Cell CycleGenes and their corresponding SNPs related to the cellular cycle were separated into three groups consisting of tumor suppressor genes, inhibitors of apoptosis, and other genes associated with BC.
Tumor suppressor genes work by repairing DNA damage, inhibiting cell division, and, in some cases, triggering apoptosis to stop tumor development. Therefore, inactivation or loss of function resulting from mutations in these genes can lead to cancer [204]. An SNP in intron 3 of the TP53 gene was associated with a decreased risk for BC in the American population [183]. Moreover, rs1042522 in the same gene is associated with an increased risk for BC in Asia [205–207]. However, this same SNP showed a protective effect towards BC in the Indian [208] and Brazilian [209] populations, and rs17878362 has the same effect in the American population [210]. Rs2839698 in H19 shows a protective effect towards BC in the Netherlands [211] whereas rs217727 in H19 [212, 213] and rs760805 in RUNX3 [214] increase BC risk in the Chinese population. Finally, rs2073636 in TSC2 is associated with a high risk for BC in the American population [172] and rs17879961 in CHEK2 with a low risk for BC in European descent [201].
Inhibitors of apoptosis are a family of eight proteins. A mutation activating any of the corresponding genes will become a weapon that will be used by tumors to evade apoptosis [215]. Rs2071214 [216], rs8073069 [216], rs9904341 [216–219], and rs3764383 [219] in BIRC5 increase BC risk, while rs17878467 decreases the risk in Asians [216, 219, 220].
Other genes include Cyclin D1 (CCND1) which regulates cell cycle progression through the activation of cyclin-dependent kinases 4 and 6 (CDK4 and CDK6) that lead to Rb protein phosphorylation, thus inactivating it and allowing the cell to progress past the G1/S checkpoint and continue its replication. Over-expression of CCND1 can lead to cancer [221] and the rs9344 in this gene increases the risk for BC [221–225]. Similarly, Cyclin E1 (CCNE1) binds to CDK2 and activates it, allowing the cell to progress and enter phase S [226]. Rs8102137 in CCNE1 widely increases BC development risk [11, 39, 73, 75, 116]. Moreover, the PI3K–AKT–mTOR is implicated in cell growth, tumorigenesis, and cell invasion [227]. Mitochondrial gene POLG polymorphism rs3087374 increases BC risk in the American population [172]. Rs2294008 [11, 37, 39, 73, 79, 228–235] and rs2978974 [70] in the prostate stem cell antigen (PSCA), an inhibitor of cell proliferation, increase widely BC risk.
Cell ArchitectureCaveolin-1 (CAV1) is an essential membrane protein expressed in multiple cells. It plays a central role in the formation of caveolae, which are small plasma membrane invaginations involved in signaling and transport. The role of CAV1 in carcinogenesis has been proven. However, the mechanism is still unknown [236]. Rs3807987 and rs7804372 in CAV1 increase the risk for BC in the Taiwanese population [237] while rs1049334 widely increases it. CLTA and CLTC, which encode the light and heavy chains of clathrin, are also associated with BC. Indeed, rs10972786 in CLTA and rs7224631 in CLTC increase the risk of BC in the European population [23]. Clathrin-mediated vesicle pathways include the DNM1 and DNM2 genes. Rs13285411 in DNM1 and rs4804528 in DNM2 increase the risk for BC, while rs4804149 in KANK2 has a significant protective effect towards BC in the European Population [23]. Finally, rs12216499 in RSPH3 widely increases the risk for BC [70], while rs907611 in LSP1 increases the risk for BC in the European population [238] but was found not to be associated with BC in Chinese [70].
Rs738141 in Dynein light chain 4 (DNAL4) implicated in cell motricity increases the risk for BC in the European population [23].
Rs16260 in Cadherin-1 (CDH1) contributes to BC susceptibility in the Chinese population [239].
The extracellular degradation process is mediated by the Matrix Metalloprotease (MMP), which plays a regulatory role in multiple pathways such as apoptosis or angiogenesis. Hence, MMPs participate in carcinogenesis [240]. Conflicting results concerning the association of rs1799750 in MMP1 with BC were obtained [241–245]. Rs243865 in MMP2 has shown an increased BC risk in India [246] and in a meta-analysis conducted by [244]. However, another meta-analysis done by L. Tao et al. has shown, for the same SNP, a protective effect towards BC in the Asian population [245]. Contradictory results have also been reported for rs11568818 in MMP7 [242, 245, 247]. Finally, rs28382575 in MMP11 increases BC risk in Taiwan [248].
MetabolicMetabolism-related genes were found to be associated with BC. These genes were divided into alcohol metabolism genes, solute carrier transporters (SLC), folate metabolism enzymes, water-soluble vitamin metabolism genes, and other various metabolism-related genes.
Alcohol metabolism requires multiple enzymes. Rs12529 in AKR1C3 decreases BC risk in the Turkish population [249] while Rs4680 in COMT has shown contradictory results [19, 250, 251].
SLC transporters are membrane proteins whose role is to supply cells with nutrients, neurotransmitters, hormones, and drugs. They are usually upregulated in cancer [252]. Rs17674580 in SLC14A [39, 253] and rs1058396 [74], rs10775480 [11, 254], rs10853535 [70, 254], rs17674580 [37, 79, 117], and rs7238033 [39, 70, 254] in SLC14A1 increase BC susceptibility in different populations. Rs1385129 in SLC2A1, also known as Glucose transporter 1 (GLUT1), decreases the carcinogenesis risk in the Chinese population [255]. Rs11871756, rs11077654, rs9913017, and rs4969054 in SLC39A11 increase the risk for BC in different populations [256] and rs2306283 in SLCO1B1 increases the risk in the Japanese population [257].
Mitochondrial folate metabolic enzymes are associated with cancer [258]. Hence, rs1667627 in MTHFD2 increases the risk of developing BC in the American population [89]. Other SNPS in genes involved in the folate metabolism are: rs1801131 [259–262] and rs1801133 [260–265] in MTHFR that are widely classified as risk factors for BC, whereas rs1476413 in the same gene decreases the risk of cancer occurrence [266], rs1805087 in MTR increases the risk for BC in the Tunisian population [262, 267] and rs1801394 in MTRR increases the risk for BC only in Saudi Arabia [18].
SNPs in the metabolism of water-soluble vitamin genes that increase BC risk include rs61330082 in NAMPT in the Chinese population [268], rs4652795 in NMNAT2, rs7636269 in NMNAT3, and rs2304191 in NMRK2 in the European population [23].
Other metabolism genes that are associated with BC are reported in Supplementary Table S1.
OthersFinally, unclassified genes that present various actions and are implicated in different pathways were also found to be associated with BC. These genes are reported under the section named “Others” in Supplementary Table S1.
Not Associated SNPsMany other SNPs in different genes were also studied and found not to modulate BC risk. Among these, rs3892097 in CYP2D6 was not found to be associated with BC in the Indian [62] and Tunisian [269] populations. Moreover, rs1800629 in TNF-α [270, 271] and rs833061 in VEGFA [122] also showed no association with BC in a meta-analysis. Rs4253211 in ERCC6 [272], rs1042489 in BIRC5 [216] and rs4880 in SOD2 [273] were also not associated with BC. Finally, rs11225395, rs35866072, and rs1940475 in MMP8 [274], as well as rs3918242 [243, 244, 275], rs3918241, rs2250889, rs17576, and rs17577 in MMP9 all showed no association with any risk for carcinogenesis [276]. Other genes and SNPs that strictly showed no association with BC risk are reported in Supplementary Table S1 under the section “No association.”
DiscussionSeveral SNPs associated with BC are restricted to specific populations. A generalization on an SNP’s potential role in predisposing or protecting an individual from BC cannot be done without testing this specific SNP in a sufficient number of individuals from different races and origins. Some SNPs were studied in different populations, which enabled us to correlate them to BC risk. On the other hand, many other SNPs that were exclusively found not to be associated with BC were only studied in one or two populations. Therefore, the scarcity of the gathered data related to these SNPs renders the interpretation of their correlation with BC challenging and requires broader studies for the validation of their contribution to this type of cancer. For SNPs with conflicting results between studies, GWAS-specific repositories could be useful.
On the other hand, the risk conferred by these variants cannot alone explain the development of BC [277]. In fact, several environmental factors were found to play a role in the pathogenesis of the disease. These include: gender, age, smoking habits, alcohol consumption, and potential environmental exposure. It is also worth mentioning that some SNPs were found to be associated with more invasiveness and recurrence. For example, PSCA rs2294008 was associated with a more invasive disease [234].
Individual SNPs effects on modulating BC risk are minimal. Therefore, studies have shifted towards assessing the polygenic risk score (PRS), as it represents a more accurate representation of an individual’s risk of developing BC. PRS aggregates the effects of multiple SNPs to provide a disease risk prediction [278]. A PRS based on 24 independent GWAS markers showed a fourfold increase in BC risk for both smokers and nonsmokers [5].
Finally, the clinical value of such information has yet to be investigated. In fact, recommendations regarding the clinical management of patients based on their genotypes are still lacking [279].
ConclusionSNPs are genetic variants that are generally population specific [8]. This review shows that several SNPs were associated with BC, depending on the studied cohorts. The generalization of the link between a variant and BC is not always possible, especially in the absence of data related to large cohorts from different ethnicities. For instance, several SNPs associated with BC are private to specific populations. On the other hand, the involvement of many SNPs in BC was ruled out based on studies focusing on one to two cohorts only. The scarcity of related data urges us to gather all the information we can in order to make it accessible to the scientific community. However, one should consider the complexity of the interpretation of these genomic markers [277], especially that, in many cases, the cumulative effect of several SNPs contributes to modulating the risk for BC [280], in addition to epidemiological or environmental factors such as gender, age, smoking habits, and alcohol consumption. Last but not least, the clinical value of such information has yet to be investigated.
Data Availability StatementThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author ContributionsAll authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of InterestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary MaterialThe Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/or.2023.10603/full#supplementary-material.
References3. Cumberbatch, MGK, Jubber, I, Black, PC, Esperto, F, Figueroa, JD, Kamat, AM, et al. Epidemiology of Bladder Cancer: A Systematic Review and Contemporary Update of Risk Factors in 2018. Eur Urol (2018) 74(6):784–95. doi:10.1016/j.eururo.2018.09.001
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Wu, K, Wang, X, Xie, Z, Liu, Z, and Lu, Y. N-Acetyltransferase 1 Polymorphism and Bladder Cancer Susceptibility: A Meta-Analysis of Epidemiological Studies. J Int Med Res (2013) 41(1):31–7. doi:10.1177/0300060513476988
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Koutros, S, Kiemeney, LA, Pal Choudhury, P, Milne, RL, Lopez de Maturana, E, Ye, Y, et al. Genome-Wide Association Study of Bladder Cancer Reveals New Biological and Translational Insights. Eur Urol (2023) 84(1):127–37. doi:10.1016/j.eururo.2023.04.020
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Audenet, F, Attalla, K, and Sfakianos, JP. The Evolution of Bladder Cancer Genomics: What Have We Learned and How Can We Use It? Urol Oncol Semin Original Invest (2018) 36(7):313–20. doi:10.1016/j.urolonc.2018.02.017
CrossRef Full Text | Google Scholar
8. Bernig, T, and Chanock, SJ. Challenges of SNP Genotyping and Genetic Variation: Its Future Role in Diagnosis and Treatment of Cancer. Expert Rev Mol Diagn (2006) 6(3):319–31. doi:10.1586/14737159.6.3.319
PubMed Abstract | CrossRef Full Text | Google Scholar
11. de Maturana, EL, Rava, M, Anumudu, C, Sáez, O, Alonso, D, and Malats, N. Bladder Cancer Genetic Susceptibility. A Systematic Review. Bladder Cancer (2018) 4(2):215–26. doi:10.3233/blc-170159
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Wogan, GN, Hecht, SS, Felton, JS, Conney, AH, and Loeb, LA. Environmental and Chemical Carcinogenesis. Semin Cancer Biol (2004) 14(6):473–86. doi:10.1016/j.semcancer.2004.06.010
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Sankhwar, M, and Sankhwar, SN. Variations in CYP Isoforms and Bladder Cancer: A Superfamily Paradigm. Urol Oncol Semin Original Invest (2014) 32(1):28.e33–28.e40. doi:10.1016/j.urolonc.2012.10.005
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Wang, Y, Kong, CZ, Zhang, Z, Yang, CM, and Li, J. Relationships Between CYP1A1 Genetic Polymorphisms and Bladder Cancer Risk: A Meta-Analysis. DNA Cel Biol (2014) 33(3):171–81. doi:10.1089/dna.2013.2298
CrossRef Full Text | Google Scholar
15. Feki-Tounsi, M, Khlifi, R, Louati, I, Fourati, M, Mhiri, MN, Hamza-Chaffai, A, et al. Polymorphisms in XRCC1, ERCC2, and ERCC3 DNA Repair Genes, CYP1A1 Xenobiotic Metabolism Gene, and Tobacco Are Associated With Bladder Cancer Susceptibility in Tunisian Population. Environ Sci Pollut Res (2017) 24(28):22476–84. doi:10.1007/s11356-017-9767-x
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Grando, JPS, Kuasne, H, Losi-Guembarovski, R, Sant’ana Rodrigues, I, Matsuda, HM, Fuganti, PE, et al. Association Between Polymorphisms in the Biometabolism Genes CYP1A1, GSTM1, GSTT1 and GSTP1 in Bladder Cancer. Clin Exp Med (2009) 9(1):21–8. doi:10.1007/s10238-008-0015-z
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Lu, Y, Zhang, XL, Xie, L, Li, TJ, He, Y, Peng, QL, et al. Lack of Association Between CYP1A1 Polymorphisms and Risk of Bladder Cancer: A Meta-Analysis. Asian Pac J Cancer Prev (2014) 15(9):4071–7. doi:10.7314/apjcp.2014.15.9.4071
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Elhawary, NA, Nassir, A, Saada, H, Dannoun, A, Qoqandi, O, Alsharif, A, et al. Combined Genetic Biomarkers Confer Susceptibility to Risk of Urothelial Bladder Carcinoma in a Saudi Population. Dis Markers (2017) 2017:1–11. doi:10.1155/2017/1474560
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Fontana, L, Delort, L, Joumard, L, Rabiau, N, Bosviel, R, Satih, S, et al. Genetic Polymorphisms in CYP1A1, CYP1B1, COMT, GSTP1 and NAT2 Genes and Association With Bladder Cancer Risk in a French Cohort. Anticancer Res (2009) 29(5):1631–5.
PubMed Abstract | Google Scholar
20. Sun, WX, Chen, YH, Liu, ZZ, Xie, JJ, Wang, W, Du, YP, et al. Association Between the CYP1A2 Polymorphisms and Risk of Cancer: A Meta-Analysis. Mol Genet Genomics (2015) 290(2):709–25. doi:10.1007/s00438-014-0956-8
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Zeng, Y, Jiang, HY, Wei, L, Xu, WD, Wang, YJ, Wang, YD, et al. Association Between the CYP1A2 Rs762551 Polymorphism and Bladder Cancer Susceptibility: A Meta-Analysis Based on Case-Control Studies. Asian Pac J Cancer Prev (2015) 16(16):7249–54. doi:10.7314/apjcp.2015.16.16.7249
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Vukovic, V, Ianuale, C, Leoncini, E, Pastorino, R, Gualano, MR, Amore, R, et al. Lack of Association Between Polymorphisms in the CYP1A2 Gene and Risk of Cancer: Evidence From Meta-Analyses. BMC Cancer (2016) 16:83. doi:10.1186/s12885-016-2096-5
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Menashe, I, Figueroa, JD, Garcia-Closas, M, Chatterjee, N, Malats, N, Picornell, A, et al. Large-Scale Pathway-Based Analysis of Bladder Cancer Genome-Wide Association Data From Five Studies of European Background. PLoS One (2012) 7(1):e29396. doi:10.1371/journal.pone.0029396
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Sankhwar, M, Sankhwar, SN, Abhishek, A, Gupta, N, and Rajender, S. CYP1B1 Gene Polymorphisms Correlate With an Increased Risk of Urinary Bladder Cancer in India. Urol Oncol Semin Original Invest (2016) 34(4):167.e1–167.e8. doi:10.1016/j.urolonc.2015.11.010
CrossRef Full Text | Google Scholar
25. Salinas-Sánchez, AS, Donate-Moreno, MJ, López-Garrido, MP, Giménez-Bachs, JM, and Escribano, J. Role of CYP1B1 Gene Polymorphisms in Bladder Cancer Susceptibility. J Urol (2012) 187(2):700–6. doi:10.1016/j.juro.2011.10.063
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Liu, Y, Lin, C, Zhang, A, Song, H, and Fan, C. The CYP1B1 Leu432Val Polymorphism and Risk of Urinary System Cancers. Tumor Biol (2014) 35(5):4719–25. doi:10.1007/s13277-014-1617-6
CrossRef Full Text | Google Scholar
27. Shi, WX, and Chen, SQ. Frequencies of Poor Metabolizers of Cytochrome P450 2C19 in Esophagus Cancer, Stomach Cancer, Lung Cancer and Bladder Cancer in Chinese Population. World J Gastroenterol (2004) 10(13):1961–3. doi:10.3748/wjg.v10.i13.463
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Basma, HA, Kobeissi, LH, Jabbour, ME, Moussa, MA, and Dhaini, HR. CYP2E1 and NQO1 Genotypes and Bladder Cancer Risk in a Lebanese Population. Int J Mol Epidemiol Genet (2013) 4(4):207–17.
PubMed Abstract | Google Scholar
29. Yin, X, Xiong, W, Wang, Y, Tang, W, Xi, W, Qian, S, et al. Association of CYP2E1 Gene Polymorphisms With Bladder Cancer Risk: A Systematic Review and Meta-Analysis. Medicine (Baltimore) (2018) 97(39):e11910. doi:10.1097/md.0000000000011910
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Deng, XD, Gao, Q, Zhang, B, Zhang, LX, Zhang, W, Er, ZEM, et al. Functional RsaI/PstI Polymorphism in Cytochrome P450 2E1 Contributes to Bladder Cancer Susceptibility: Evidence From a Meta-Analysis. Asian Pac J Cancer Prev (2014) 15(12):4977–82. doi:10.7314/apjcp.2014.15.12.4977
Comments (0)