Questions about the specific properties of the human brain originated in the debates that followed the publication of Darwin (1859)'s Origin of Species. Challenging long-held beliefs, largely inherited from the biblical Genesis account, that humans were by essence different from other animals, the application of the theory of evolution to humans soon gave rise to lively discussions about the biological traits likely to differentiate humans from apes and stimulated the first thoughts about the relationship between brain size and cognitive ability. Considered a good indicator of intelligence by most scientists in the second half of the 19th century, overall brain size eventually proved to be an irrelevant measure of behavioural complexity, prompting the exploration of other levels of brain organisation. Thanks to in vitro investigations on postoperative tissue, our knowledge of the human neocortex—the cerebral structure whose functioning is critically involved in behavioural and cognitive abilities—has progressed considerably in recent years, providing the basis for inter-species comparisons at multiple levels. After tracing the historical origins of these questions back to the controversies surrounding the first discoveries of human fossils, this review is intended to provide an updated view of the functional organisation of the neocortex in representatives of the three major mammalian radiations, including humans. We will first analyze variations in brain size, number of cortical areas and number of neurons at a macroscopic level, before focusing on the anatomical and physiological features of neocortical pyramidal neurons. Adopting a reductionist approach, we will systematically compare (when data permit) the morphology, connectivity and electrical properties of pyramidal neuron subtypes between species, attempting to determine, where differences are observed, whether they are part of a continuum of variations or whether they represent genuine singularities leading to significant changes in the activity patterns of cortical circuits.
2 Eugene Dubois and the missing linkProponents of Darwin's theory, led by the German biologist Ernst Haeckel, published essays in the 1860s discussing the status of the human species from an evolutionary point of view. According to Haeckel, the process of hominization was based on the acquisition of bipedalism, language and a large brain. In his family tree of the human species, which he attempted to reconstruct on the basis of comparative anatomical and embryological data, Haeckel inserted an intermediate evolutionary stage between the great apes and man, occupied by a hypothetical species called Pithecanthropus (ape-man) alalus (speechless). He imagined that this mute ape-man, originating from a continent now sunk in the Indian Ocean (Lemuria), could have spread and evolved in different parts of the world to give rise to different humanities and languages (Haeckel, 1868). Inspired by the work of Darwin and Haeckel and convinced of the necessity to support the theory of evolution with palaeontological evidence, in 1887 the young anatomist Eugène Dubois took the surprising decision to quit a promising academic career at the University of Amsterdam to mount an excavation campaign in the Dutch East Indies in search of the missing link between apes and humans. Accompanied by his wife Anna Lojenga and their daughter, Dubois enlisted as a medical officer in the Royal East India Army and set sail for the Indonesian archipelago aboard the Princess Amelia (Theunissen, 1988; Wood, 2020).
After arriving on the island of Java, Dubois conducted extensive excavations in the summer of 1891 near the village of Trinil, along the Solo River (Figure 1). He initially uncovered a right maxillary third molar and a skull cap whose characteristics—a receding forehead, a supra-orbital torus and an estimated capacity of 700–750 cm3 (about half the size of present-day humans)—suggested a large ape. However, the next year, Dubois unearthed a left femur some 15 metres upstream from the first remains, showing clear adaptations to upright posture and bipedalism (Theunissen, 1988). By discovering an individual whose skull and teeth displayed anthropoid ape characteristics but whose femur showed human-like features, Dubois had just uncovered fossil evidence of the hypothetical transitional primate envisioned by Haeckel, and at the same time provided one of the first material indications of human evolution. Initially named Anthropithecus erectus (an ape that stands and moves like a man) in the excavation reports, Dubois later renamed his new species Pithecanthropus erectus, emphasising its status as an upright “ape-man,” when he published his final manuscript (Figure 1). In good faith, he even revised the cranial capacity of his specimen to 850–900 cm3 (Dubois, 1894, 1896; Wood, 2020).
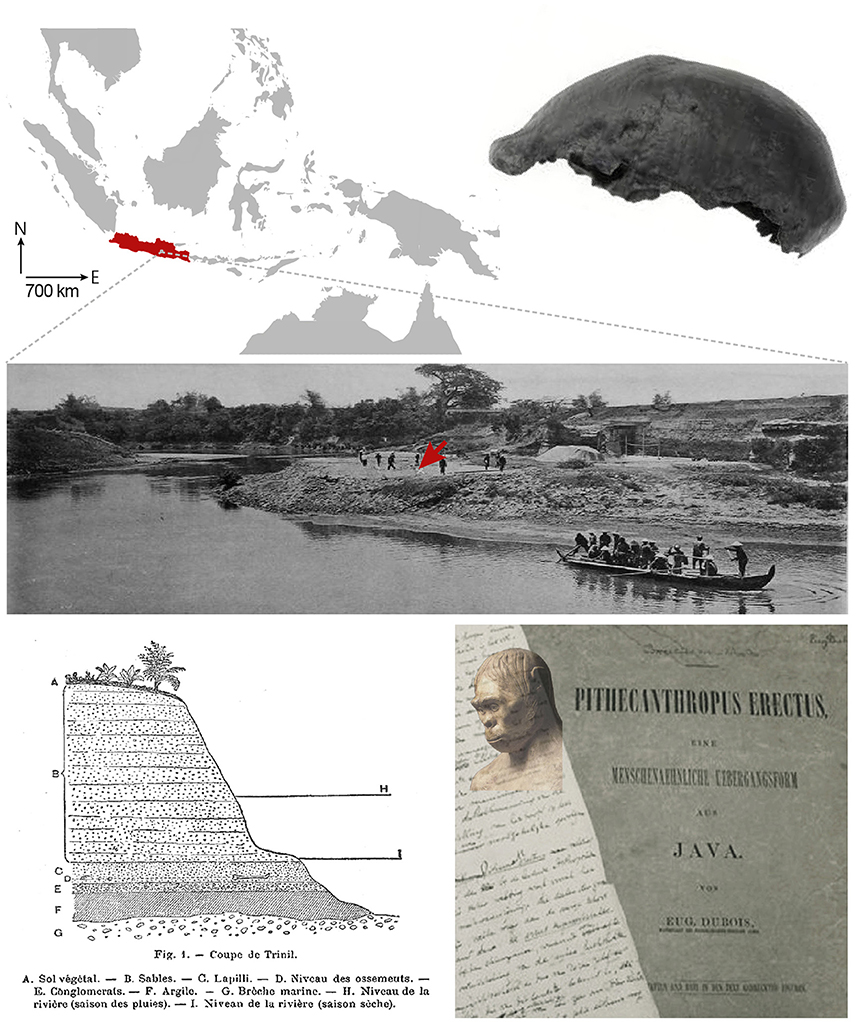
Figure 1. Top left, The Island of Java in the Indonesian archipelago. Right, The skull cap of Pithecanthropus erectus. Middle, Excavations at Trinil in 1890s on the left bank of the Solo River. The arrow indicates the approximate discovery site of the skull cap. Bottom left, Profile drawing of the Trinil site showing the position of the fossil remains in the sediment (level D). Right, Handwritten page from Dubois' article published in 1894 and photograph (John Reader/Science Photo Library) of his reconstruction of Pithecanthropus erectus, shown at the 1900 Universal Exhibition in Paris. Adapted with permission from Wood, 2020, and Dubois, 1896.
The discovery of Pithecanthropus erectus sparked controversy, particularly over the attribution of the remains to an ape, a human, an intermediate being, or even to different individuals. Dubois had to face the scepticism of his prominent European colleagues who would have preferred an ancestor with a larger brain but less exotic origins (Leguebe, 1992), such as the Piltdown Man discovered in Sussex in 1912, which had a large human-like skull and an ape-like mandible, but which ultimately turned out to be one of the greatest paleoanthropological hoaxes (Stringer, 2012). In response to debates over the interpretation of his fossils, Dubois undertook research on the allometric relationship between brain weight and body weight. Extending earlier theoretical works (Snell, 1892), he established that brain size was not only related to body weight by a decreasing power function but also depended on a “coefficient of cephalization,” supposed to reflect the degree of development and complexification of the brain (Dubois, 1897). Applying this mathematical relationship to his fossils, Dubois calculated that the cephalization coefficient of Pithecantropus erectus was roughly half that of anatomically modern humans and double that of apes, further confirming the intermediate evolutionary position of the Javanese primate (Dubois, 1899).
Pithecanthropus erectus is no longer considered the missing link. The idea of a hybrid creature, half ape and half human, making a direct transition between great apes and modern man, now belongs to the realm of fiction. The accumulation of fossil discoveries has led researchers to abandon the traditional vision of a linear and directed human evolution in favour of a more complex and diversified human lineage, made up of multiple species that most often coexisted. The concept of the missing link, which encompasses both the notion of continuity and rupture, remains pertinent as it questions the singularity of the “after” in relation to the “before.” Man is often seen as a species apart, distinguished by the complexity of its cultures, social interactions and ability to communicate. A species whose considerable brain growth over time would have accompanied the emergence of remarkable cognitive capacities, enabling man to conquer and transform almost all of the terrestrial ecosystems. But the question remains: does our large brain possess truly distinctive properties? And if so, are these differences merely quantitative or do they represent a genuine qualitative leap?
3 The long-standing question of sizeQuestions of absolute or relative brain size seem to have preoccupied mankind long before Dubois and his contemporaries since they already appear in the writings of Aristotle, who notes that “of all animals, man has the largest brain in proportion to his size” (Aristotle, ca. 335 BCE); a statement that is not entirely accurate, as we shall see below. However, it was mainly in the second half of the 19th century that the relationship between brain size and human cognitive ability became a central theme of discussion among the scientific community, particularly within the Société d'Anthropologie de Paris, founded by the eminent neuroanatomist Paul Broca the year Darwin published his Origin of Species. A partisan of polygenist theories, which, in opposition to the creationist myth, favoured a multiple origin for the different human groups, but unfortunately mired in the prejudices of his time, Broca mistakenly thought that he could rank ethnic groups (and human beings in general) according to their level of intelligence by comparing the weight of their brains. By sorting individuals according to their ethnic origin, sex or profession, Broca came to the conclusion that white European men, whom he described as “distinguished” (as opposed to manual workers), were endowed with superior intelligence (Broca, 1861). In a similar vein, Francis Galton, an anthropologist renowned for his contributions to modern statistics and, more infamously, for his eugenics theories, later conducted a study into the brain size of Cambridge students. He also claimed, on the basis of measurements of questionable rigour, that those who graduated with honours had larger brains than those who did not receive such distinction (Galton, 1889).
In the wake of phrenology, which claimed to determine character traits and mental faculties by inspecting the size of bumps on the surface of skulls, the underlying—perhaps somewhat simplistic—assumption behind these early attempts to explain differences in cognitive ability by brain size was that any increase in the size of an organ should correspond to an increase in its function. Craniometric studies based on the social or geographical origins of human beings, which served as scientific justification for the expansionist ambitions of many countries in the 19th and 20th centuries, fortunately declined after the Second World War. However, the search for principles behind the evolution of the mammalian brain has never ceased to intrigue scientists, who continue to study variations in absolute and relative brain size between species, as well as the evolution of its different parts, paying particular attention to the neocortex because of its essential role in the generation of complex behaviours.
3.1 Brain size in mammalsAs neuronal tissue does not fossilise, the formulation of general principles on the evolution of the brain requires a comparative analysis of cerebral organisation in living species (and, to a lesser extent, the study of endocranial casts of fossil specimens), based on the hypothesis that the characteristics present in the current members of a phylogenetic radiation can be explained more parsimoniously as being inherited from a common ancestor. The earliest mammals have likely evolved from mammal-like reptiles at the end of the Triassic over 200 million years ago, in the form of small-brained shrew-like creatures that probably laid eggs, like present-day monotremes (Kaas, 2011). Monotremes (prototherians), one of the three main extant mammalian groups, diverged from therians around 166–186 million years ago, while the marsupial (metatherian) and placental (eutherian) lineages are thought to have split around 147–160 million years ago (Bininda-Emonds et al., 2007; Phillips et al., 2009).
The brain size of present-day mammals is extremely variable, ranging from <0.1 g in the Etruscan pygmy shrew to more than 9 kg in some large cetaceans (DeFelipe, 2011). In placentals, evolutionary processes have led to the emergence of large brains in several groups of species sometimes separated by a long independent phylogenetic history (Manger et al., 2013). These include a large proportion of whales (up to 9,200 g for the sperm whale) and dolphins (up to 2,900 g), elephants (up to 6,000 g), certain pinniped species (such as walruses or southern elephant seals ~1,200 g), and members of the genus Homo (see Figure 2). Modern Homo sapiens, with an average brain mass of 1,350–1,400 g (varying from 1,100 to 1,800 g), are a long way behind elephants and cetaceans, but can nevertheless claim first place among primates, since the largest brains of the great apes do not exceed 500–600 g (Tower, 1954; Jerison, 1973; Haug, 1987; Roth and Dicke, 2005; Neubauer et al., 2018). Differences in brain size in present-day Homo sapiens do not appear to have functional significance, since substantial variations can be observed between individuals with apparently similar intellectual abilities (DeFelipe, 2011). The idea that a larger absolute brain size should necessarily confer more complex cognitive abilities or greater behavioural flexibility is also challenged by observations that animals with similar brain weights, such as gorillas and oxen (~500 g) or elephant seals and some humans (~1,200 g), have different behavioural repertoires, or that species with relatively small brains, such as dogs (60 g), rats (2 g) and mice (0.5 g) in mammals or corvids (6–15 g) in birds, can demonstrate sophisticated behaviours (Kaminski et al., 2004; Olkowicz et al., 2016).
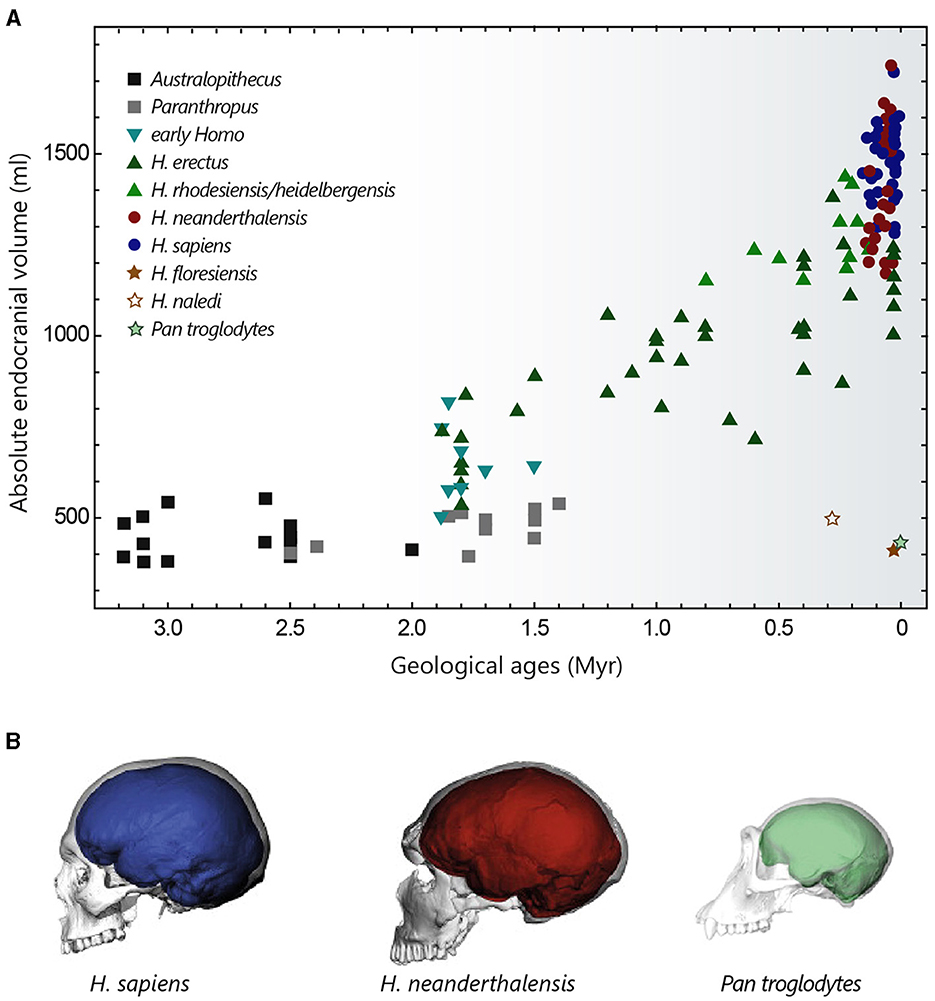
Figure 2. Evolution of hominin brain size. (A) Evolution of hominin endocranial volume over time. Adapted with permission from Hublin et al. (2015). (B) Differences in brain size and shape of a modern human (blue), a Neanderthal from La Chapelle-aux-Saints (red) and a chimpanzee (green), visualised by computer tomography. Adapted with permission from Neubauer et al. (2018, 2020).
Another widely studied property is relative brain size. In mammals, brain size and body size are closely correlated, with a negative allometry. As a result, small species tend to have relatively larger brains in proportion to their body size than large species. In humans, for example, the brain represents around 2% of body mass, whereas this ratio can reach 10% in shrews and small rodents (Van Dongen, 1998; Roth and Dicke, 2005). It is this negative scaling relationship that led Dubois to introduce the “coefficient of cephalization,” later renamed the encephalization quotient (EQ), as a suitable measure for comparing relative brain size across species (Jerison, 1973). The EQ quantifies how much a species brain size deviates from what is expected based on its total body mass, using a standard species from the same taxonomic group as a reference. When calculating EQs in mammals, using the cat as the standard, humans emerge as the most encephalized species with an EQ of around 6–7, meaning that their brains are more than six times larger than expected. However, a high degree of encephalization is not exclusive to humans. Dolphins like the Tucuxi or the white-sided dolphin are not far behind humans with EQs around 4–5, followed by capuchin monkeys with EQs ranging from 2.4 to 4.8. Then come species like gorillas and chimpanzees (EQ = 1.5–3)—though often considered more cognitively able than capuchin monkeys (Deaner et al., 2007), elephants (EQ = 1.1–2.4), and rodents with relatively low EQ values of 0.5–1 (Jerison, 1973; Marino, 1998, 2002; Roth and Dicke, 2005; Shoshani et al., 2006). However, the ability to predict the cognitive abilities of a species from EQ values remains quite limited, mainly due to the sensitivity of this metric to the choice of the reference taxonomic group and to the value of the exponent of the power law relating brain size to body size (typically between ~0.55 and 0.8) used in the various studies (Harvey and Krebs, 1990; Marino, 1998; Charpier, 2008).
3.2 Brain size in homininsThe cranial capacity of hominins has undergone a significant increase over the past 3 million years, evolving from 450 cm3 in Australopithecus (a value similar to that of the great apes) to approximately 1,350–1,500 cm3 in Homo sapiens and Homo neanderthalensis (that went extinct ~40,000 years ago) (Figure 2A) (Jerison, 1973; Holloway, 2015; Neubauer et al., 2018). This cerebral expansion, particularly evident in Homo erectus (~2 million years ago), was initially closely linked to changes in body weight and then progressed more independently over the last 500,000 years (Ruff et al., 1997; Hublin et al., 2015), explaining the high EQ values of Homo sapiens. It is interesting to note that Homo floresiensis, whose remains were discovered in the Liang Bua cave on the island of Flores, represents an exception to this brain size expansion in the Homo lineage. Despite its ability to create sophisticated tools and perhaps to navigate, Homo floresiensis (dated between ~100,000 and 50,000 years ago) had unexpected morphological features such as a small stature (~1 metre) and a small endocranial volume of around 430 cm3 (Balzeau and Charlier, 2016; Sutikna et al., 2016). Similarly, Homo naledi, a new species recently discovered near Johannesburg in South Africa, which coexisted with Neanderthals and potentially with the first Homo sapiens, also possessed small brain capacities ranging from 465 to 610 cm3 (Garvin et al., 2017). These fossil discoveries are profoundly challenging our knowledge of human diversity and the long-held idea of a continuous evolution towards ever-larger human beings with ever-more-developed brains.
The increase in size of the hominin brain over the course of evolution entails an increase in its energy cost. The metabolic requirements of a human brain are considerable: accounting for 50–55% of basal metabolism at birth, this proportion peaks at over 65% at the age of 4–5 years and remains high, at around 20%, in adulthood (Clarke and Sokoloff, 1999; Kuzawa et al., 2014). It is believed that the consumption of energy-rich foods like meat and marrow, and, later, their improved digestibility through cooking (Carmody and Wrangham, 2009), have enabled mothers to allocate more energy to their foetuses during pregnancy (and their newborns during nursing) and have thus favoured the development of larger brains (Martin, 1996). Thus, the large brains of early Homo may have emerged as an unintended by-product of a change in maternal diet, perhaps initiated by a modification in climate and available resources. Another hypothesis suggests that a reallocation of energy to the brain may have been facilitated by a reduction in the size of other metabolically costly organs, such as the digestive system (Aiello and Wheeler, 1995). Finally, the social brain hypothesis proposes that the evolution of hominin encephalization could be the result of increasingly complex social demands in group-living species (Dunbar and Shultz, 2007).
Compared with chimpanzees and macaques (~40 and 70% brain growth in utero, respectively), a significant proportion of brain growth in humans occurs after birth. The size of the brain at birth is thought to be partly constrained by the anatomy of the woman's pelvis, whose dimensions are limited by biomechanical and postural factors associated with bipedalism (Hublin et al., 2015). From a size of around 400 cm3 at birth (~28% growth in utero), the brain of a young Homo sapiens undergoes rapid growth during the 1st year, by the end of which it reaches 50% of its adult size, finally attained at the age of 5–6 years (Leigh, 2004; DeSilva and Lesnik, 2006). This perinatal phase of brain growth is accompanied by a change towards a more globular shape, typical of modern humans. This globularization process, which evolved progressively to reach the current variation between 100,000 and 35,000 years ago, is not observed in Neanderthals and chimpanzees (Figure 2B) (Bruner et al., 2003; Gunz et al., 2010; Neubauer et al., 2010, 2018).
3.3 The neocortical expansionWhile a simple cortex is already present in the pallium of reptiles, the neocortex first appears as a complex multi-laminated structure in mammals (Nieuwenhuys, 1994; Rakic, 2009). Of modest proportion (20–48%) in small species of the three main radiations, such as platypuses, opossums, shrews and mice, it reaches considerable size in cetaceans and primates. Accounting for 75 to 84% (depending on the study) of the total mass or volume of the brain, the human neocortex is proportionally one of the largest among mammals. Humans are however closely followed (and sometimes equalled) by most odontocete cetaceans (~72%), as well as several species of monkeys and apes, such as macaques (70–76%), grivets (79%), or chimpanzees (70–73%) (Pirlot and Nelson, 1978; Stephan et al., 1981; Hofman, 1988; Rilling and Insel, 1999; Manger, 2006). Within the neocortex, a pronounced enlargement of the frontal lobe, thought to be involved in higher cognitive functions, has long been regarded as a hallmark of human evolution. Brodmann (1912), shortly after completing his famous cytoarchitectural map of the cerebral cortex, published a comparative study of the frontal cortex surface in primates, demonstrating a progressive increase from prosimians to humans. While more recent studies have indeed demonstrated an increase in the absolute size of the frontal cortex in humans (Semendeferi et al., 1997, 2002; Bush and Allman, 2004), this expansion does not seem to significantly differ from what would be expected from a great ape with a human-sized brain (Semendeferi et al., 2002; Barton and Venditti, 2013).
Neocortex thickness generally correlates positively with brain size (Hutsler et al., 2005; Balaram and Kaas, 2014). However, this correlation does not uniformly apply to all taxa, as evidenced by the typically thin cortex (<2 mm) of cetaceans (Ridgway and Brownson, 1984). The average variation in cortical thickness between species (between 0.4 and 2.8 mm), which is comparable to the variability found between cortical areas in the same animal, remains relatively modest compared to the variation in overall brain size. This suggests that the expansion of the neocortex in large-brained mammals is mainly the result of an increase in its surface area (rather than its thickness), often resulting in the formation of convolutions, particularly visible in large primates and cetaceans (Hofman, 1985, 1988; DeFelipe, 2011 for review). In primates, a gradual increase in neocortical thickness is observed from primary to more integrative sensory areas, a trend seemingly absent in motor and frontal association cortices (Wagstyl et al., 2020). Finally, the relative thickness of cortical layers also varies between species, with supragranular layers being proportionally thicker in primates than in carnivores and rodents, while infragranular layers show an inverted profile (Hutsler et al., 2005).
4 Intrinsic organisation of the neocortex 4.1 Areas and columnsThe mammalian neocortex is typically subdivided into six layers defined by vertical differences in the size, shape, or density of neurons (Brodmann, 1909). There are, however, variations in the number, thickness or overall cytoarchitectonic organisation of the layers across the cortical mantle (Kaas, 1987; DeFelipe, 2011), which have formed the basis of its subdivision into distinct regions.
The neocortex is classically regarded as a complex mosaic of anatomically and functionally specialised areas whose number increases with brain size (Brodmann, 1909). From about 10 to 15 cytoarchitectonic subdivisions mainly dedicated to sensory processing (primary somatosensory, visual, and auditory areas) and motor functions (although controversy remains over a clear separation of somatosensory and motor regions in some early mammals) in small-brained species (Krubitzer et al., 1995; Catania et al., 1999; Kaas, 2011), their number could approach 200 in humans (Glasser et al., 2016). In large-brained species, primary sensory and motor fields subdivide—more than 10 to 20 areas identified for the single visual cortex in cats and monkeys—, change in size and relative position and become separated by the inclusion of associative areas notably in the frontal and temporo-parietal regions (Nieuwenhuys, 1994; Northcutt and Kaas, 1995). The small amount of cortical territory devoted to multimodal or association areas in monotremes suggests that unimodal sensory fields could constitute the core of the prototypical plan for neocortical organisation in mammals (Krubitzer et al., 1995). The developmental mechanisms responsible for this elaborate cortical parcellation have been debated as to whether the structural differences between areas are induced in a homogeneous population of cortical neurons by the patterned activity of thalamocortical projections, or whether the formation of the neocortical map is already genetically determined in the neural progenitors of the embryonic ventricular zone. In this later view, which seems to be gaining consensus, areas in the cortical plate would attract appropriate inputs rather than being specified by them. Activity-dependent mechanisms would then play an influential role at later stages in refining existing synaptic connexions (Rakic, 2002).
The predominance of vertical over horizontal connexions in his anatomical reconstructions of rodent cortical neurons, led Lorente de Nó (1938) to suggest in the 1930s that cortical areas were composed of multiple 'elementary units' of information processing taking the form of vertical bands of interconnected neurons. Almost 20 years later, Mountcastle obtained persuasive evidence of a columnar segregation of sensory modalities in the cat somatosensory cortex by showing that neurons recorded along different vertical microelectrode tracks responded either to superficial or deep cutaneous stimulation. He introduced the term 'cortical column', assigned them an average width of ~0.5 mm and demonstrated that the different functional columns were intermingled in the manner of a mosaic (Mountcastle, 1957). Columnar organisation formed by groups of neurons activated more strongly by stimulation of one of the two eyes (ocular dominance column) or by stimuli having a common receptive field axis orientation (orientation columns) were later discovered in the cat primary visual cortex (Hubel and Wiesel, 1963, 1969). Comparing data obtained in cats and macaques, Hubel and Wiesel (1963, 1974) found that similar variations in orientation tuning were obtained with smaller electrode advances in monkeys, suggesting thinner columns or less defined borders in this species. Variations in the width of ocular dominance columns (from 200 to 800 μm) were also reported in subsequent studies on different primate species including humans (Bugbee and Goldman-Rakic, 1983; Horton and Adams, 2005), finally leading to the introduction of a new entity, the minicolumn, whose iteration and lateral combination through short-range horizontal connexions would form the basis of functional columns. Developmentally, minicolumns reflect the radial migration of neurons from the proliferative ventricular zone into narrow (30–50 μm) translaminar chain of cells separated by neuropil (Buxhoeveden and Casanova, 2002; Rakic, 2002). According to the “radial unit hypothesis,” the surface expansion of the neocortex during mammalian evolution (by ~10,000 times from shrews to the largest cetaceans; Hofman, 1985; Manger, 2006), with no comparable variation in its thickness, could result from a change in the genetic mechanisms that control the timing and/or mode of cell division in the ventricular zone, leading to an increase in the pool of founder cells at the origin of radial columns (Rakic, 1995; Chenn and Walsh, 2002).
Anatomical and functional evidence for a modular organisation of the neocortex has been obtained in a wide range of species from different mammalian radiations. Alternating bands of corticocortical projections related to monoaural or binaural responses are observed in the cat primary auditory cortex (Imig and Adrián, 1977), and patchy arrangements of axon terminal fields are apparent in the auditory area of the short-beaked echidna (Dann and Buhl, 1995). An additional example is the discrete architectonic units, known as “barrels,” formed by neurons preferentially activated by the same facial whisker in the primary somatosensory cortex of rodents and several other mammals with whiskers (Woolsey and Van der Loos, 1970). In the platypus somatosensory area, regions where neurons respond only to cutaneous stimulation of the bill are separated from regions where neurons process both tactile and electrical inputs (Krubitzer et al., 1995). Most frequently observed in primary sensory systems, the presence of columns is also attested in primary motor and association cortices (Bugbee and Goldman-Rakic, 1983; Amirikian and Georgopoulos, 2003). The existence of relatively similar organisational patterns in various areas and species led to idea that modular units could represent a fundamental principle of cortical function in mammals, important for perception, cognition and memory (Eccles, 1981; Mountcastle, 1997). In this context, it is expected that the subdivision of cortical regions into iterated computational units capable of operating in parallel should increase the number of possible spatio-temporal combinations of activity, and hence the processing capacities of large brains. However, the concept of cortical columns has also been contested based on an apparent intra- and inter-species inhomogeneity in size, shape, and expression without obvious differences in cortical function. For example, ocular dominance columns are well defined in Old World monkeys and remain rudimentary in most New World monkeys (Hendrickson et al., 1978; Adams and Horton, 2003), despite similar visual abilities. Similarly, barrels are not found in all the marsupial species that possess whiskers, and some rodents, like the chinchilla, have barrel fields without engaging in whisking behaviour (Purves et al., 1992). These findings raise the possibility that cortical modules may have emerged in different forms during areal specification in mammals, without acquiring an obvious function in all species (Horton and Adams, 2005).
4.2 Neurons and synapses in numbersThe mammalian neocortex contains approximately 15–25% of the total number of brain neurons (Azevedo et al., 2009; Herculano-Houzel, 2012). Initial assumptions that cortical columns were composed of a constant number of neurons in all mammals (Rockel et al., 1980) have been challenged by subsequent studies showing, using stereological and non-stereological counting methods, variations in the density of neurons between species, areas and layers (DeFelipe et al., 2002; Herculano-Houzel et al., 2008). Neuronal density in the neocortex generally tends to be inversely correlated with brain volume, with different scaling rules applying to different orders of mammals. Thus, for a similar increase in neocortex mass, the corresponding decrease in neuronal density seems to be less pronounced in primates than in other placental mammals (Haug, 1987; Manger, 2006; Herculano-Houzel, 2012; Herculano-Houzel et al., 2014), and marsupials are reported to have fewer neurons than placentals of equivalent brain size (Haug, 1987; Seelke et al., 2014). The total number of cortical neurons in the human brain (12–16 billion) therefore exceeds that measured in other large-brained species such as whales or elephants (6–11 billion), but this number aligns with expectations for a primate with a human-sized brain (Haug, 1987; Azevedo et al., 2009; Herculano-Houzel et al., 2014). However, Pinson and colleagues recently discovered that expression of the modern human variant of transkelotase-like protein 1 (hTKTL1)—but not of the Neanderthal variant (which differs by a single amino acid substitution)—in the embryonic mouse neocortex can increase the abundance of a specific type of basal progenitors and promote neuron production, especially in the frontal lobe. These findings suggest that, even within primates, species with similar brain sizes, such as Homo sapiens and Neanderthals, may exhibit variations in the number of neurons (Pinson et al., 2022).
Differences in counting methods, variations in age and number of samples, or in the amount of cortical volume examined make it difficult to compare calculations of synaptic density between laboratories (DeFelipe et al., 2002). Nevertheless, most studies agree that the mean synaptic density in the adult (defined as the total number of excitatory and inhibitory synapses per unit volume of cortical tissue) do vary across species (between ~250 and 1,000 million/mm3), but relatively independently of brain size (see DeFelipe et al., 2002; Karbowski, 2014 for reviews). For instance, a recent comparative study conducted in 25 primate species (including humans) found relatively constant synaptic densities in the primary visual and inferior temporal cortex of the different animals (~256 million/mm3), varying by only 1.9-fold despite brain weights differing by about 500-fold (Sherwood et al., 2020). Although a certain percentage of synapses continue to be remodelled in adulthood, synaptic density in the adult neocortex is globally stationary. This period of stable synaptogenesis is preceded by major changes in the rate of synapse production, which is particularly high during the perinatal period. The duration of this massive increase in synapse density around birth varies widely between mammals, ranging from 2 weeks in rats, 1 month in cats, 4 months in macaques, to around 3 years in humans (Bourgeois, 2008). The number of synapses is then maintained at a maximum until puberty (which is delayed in primates compared with rats and cats), during which synaptic density decreases markedly to levels comparable to those observed in adults (Huttenlocher, 1979; Bourgeois and Rakic, 1993; Bourgeois, 2008; Elston and Fujita, 2014). The maturation period for synaptic architecture is therefore considerably lengthened in primates, suggesting that the sensory environment could play an important role in the configuration and refinement of cortical circuits.
The number of synapses per neuron, usually estimated by dividing the synaptic density by the neuronal density in a given layer, positively correlates with brain volume. In primates, the overall number of synapses per neuron (including both excitatory and inhibitory cells) in the inferior temporal cortex is thus higher in humans (~4,850 synapses/neuron) than in gorillas (~3,550 synapses/neuron), chimpanzees (~2,885 synapses/neuron), and macaques (~2,160 synapses/neuron) (Sherwood et al., 2020). However, this positive scaling does not universally apply to all species, as sensory cortex neurons in mice are reported to have more synapses than in rats (DeFelipe et al., 2002). The ratio of synapse density to neuron density remains a relatively coarse measure of connectivity because it does not differentiate between different types of neurons and overlooks the fact that dendrites, particularly those of pyramidal neurons, generally span several layers. A more accurate estimate of the number of synapses received by a given neuron could perhaps be obtained by quantifying the spine density along small dendritic segments (assuming that each dendritic spine is contacted by at least one synaptic input) and extrapolating these measurements to a cumulative number of spines, considering the length of the different dendritic compartments. Such analyses revealed that the density of spines on pyramidal neurons from the supragranular layers of the temporal cortex is higher in humans than in macaques (1.35 times), marmosets (1.9 times), or mice (1.3 times) (Elston et al., 2001; Benavides-Piccione et al., 2002). Based on these calculations and measures of total dendritic length, the total number of synapses received by a human temporal cortex L2/3 pyramidal cell has been estimated to be around 20,000 (Eyal et al., 2018).
4.3 Constituent cell types and functional microcircuit organisationCortical circuit computations rely on the dialogic interaction of two main classes of neurons: the spiny glutamatergic excitatory neurons (comprising pyramidal and stellate cells), processing and transmitting information within and/or outside the neocortex, and the smooth or sparsely spiny GABAergic inhibitory interneurons, which finely regulate synaptic activity of local populations of excitatory neurons, shaping network dynamics.
The following sections will primarily address the structural characteristics of the pyramidal neuron, accounting for ~70–80% of the total population of neocortical neurons in placental mammals. Qualified as the “psychic cells” of the brain by Santiago Ramón y Cajal, pyramidal neurons are distributed across all cortical layers (except L1, where they still extend dendrites), and are regarded as the cornerstone of the cortical microcircuitry. The typical eutherian mammalian pyramidal neuron is distinguished by its prominent apical dendrite, radially oriented towards the pia, and its skirt of basal dendrites radiating from the soma (Figure 3). Pyramidal cells can be broadly classified as intratelencephalic (IT) or extratelencephalic (ET), depending on whether their long-range axons are confined to telencephalic structures (such as the neocortex, striatum, or claustrum) or whether they additionally establish connexions with brain structures outside the telencephalon (thalamus, tectum, pons, spinal cord). IT neurons are distributed throughout layers 2 to 6, while ET cells are confined to the deeper layers 5–6 (Harris and Shepherd, 2015; Baker et al., 2018a for reviews). IT neurons are the sole source of interhemispheric connexions, conveyed through the anterior commissure in monotremes and marsupials, as well as through the corpus callosum in eutherians (Suárez et al., 2018). Spiny stellate cells, lacking a prominent apical dendrite and instead featuring a star-like dendritic arbour, are predominantly localised in L4 of primary sensory cortices. Below I present an overview of the organisation of cortical circuits, outlining the main classes of neurons and their input-output connectivity patterns. This description is mainly based on findings obtained in eutherian mammals (in particular rodents, cats, and monkeys), with attempts to draw comparisons with the monotreme and marsupial literature where feasible. I will not cover here the properties of cortical astrocytes, which are now recognised as key contributors to various neuronal functions, including synaptic transmission, energy metabolism, and ion homeostasis. However, investigating their diversity and morpho-functional features within the main mammalian groups represents a promising direction for future research, given the reported variations in the number or size of protoplasmic astrocyte processes between humans and rodents, as well as the specific presence of certain astrocyte types in primates (see Oberheim Bush and Nedergaard, 2017 for review).
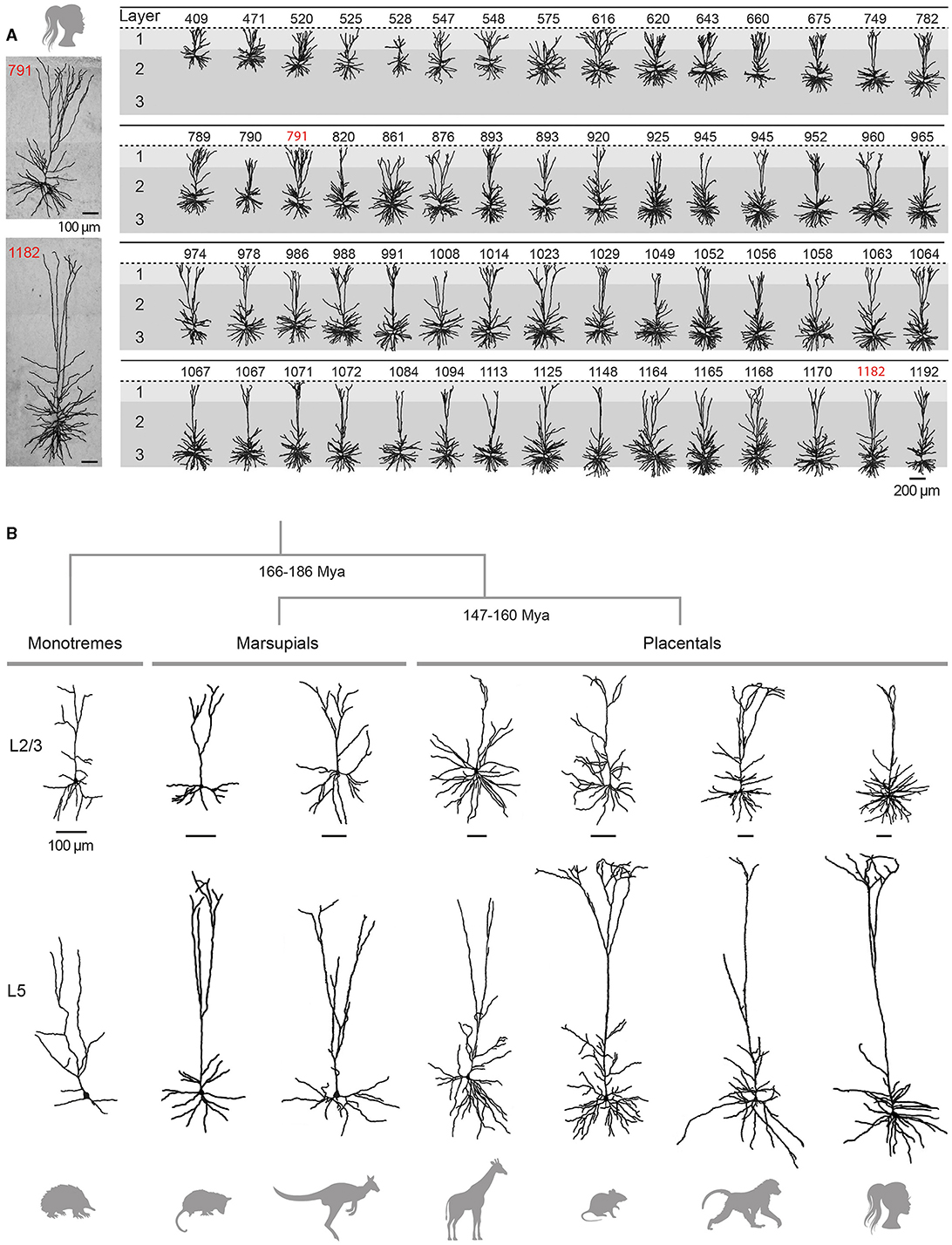
Figure 3. Variability and evolution of pyramidal neuron morphology in mammals. (A) Database of reconstructed human temporal cortex L2/3 neurons, classified according to their somatic depth, indicated in μm at the top of each cell. Two examples of cells whose soma was located 791 (top) and 1182 (bottom) μm from the cortical surface are enlarged on the left. Adapted with permission from Deitcher et al. (2017). (B) Examples of supragranular (L2/3, top) and infragranular (L5, bottom) pyramidal neurons from the visual (L2/3) and motor (L5) cortices of the short-beaked echidna, the primary motor cortex of Benett's wallaby, the sensorimotor cortex of the American opossum, the primary motor cortex of the northern giraffe, the primary somatosensory cortex of the Sprague-Dawley rat, the primary motor cortex of the chacma baboon and the human temporal cortex. Adapted from Dann and Buhl (1995) (echidna L2/3); Hassiotis and Ashwell (2003) (echidna L5), Jacobs et al. (2018) (wallaby, baboon, giraffe), Boyer et al. (2022) (rat L2/3), Mahon and Charpier (2012) (rat L5), Deitcher et al. (2017) (human L2/3), and Kalmbach et al. (2021) (human L5). Neurons were redrawn from the sources mentioned. Scale bars (100 μm) are species-specific and apply to supra- and infragranular neurons. Estimates for monotreme-therian divergence and marsupial-placental separation are taken from Bininda-Emonds et al. (2007) and Phillips et al. (2009).
4.3.1 Upper-layer pyramidal neurons in eutheriansL2/3 pyramidal neurons, a subset of IT neurons, play a pivotal role in intra-cortical information processing through their local and long-range corticocortical connexions. These neurons receive inputs from specific thalamic nuclei on their basal dendrites, either directly or via ascending projections from L4 in sensory cortices or from upper L5 in cortices lacking a distinct granular layer, while non-specific thalamocortical and distant cortical inputs mainly terminate on their tuft branches in L1. L2/3 pyramidal neurons make numerous reciprocal connexions within their home column, mostly on upper basal and apical oblique dendrites. Locally, L2/3 ITs send prominent descending axonal projections to L5 pyramidal cells (Weiler et al., 2008; Lefort et al., 2009; Petreanu et al., 2009; Harris and Shepherd, 2015). This robust output to L5 has been identified as an essential feature of neocortical microcircuitry, preserved in most regions and species (Thomson and Lamy, 2007; Weiler et al., 2008; Hooks et al., 2011). Long-range axons of supragranular pyramids establish connexions with ipsi- and contralateral cortical regions and the striatum (Petreanu et al., 2007; Anderson et al., 2010; Pidoux et al., 2011a).
L2/3 pyramidal neurons show substantial regional and inter-species variation in their dendritic architecture. In primates, L2/3 ITs from higher integrative frontal cortices generally display a more extensive and branched basal dendritic tree than their counterparts from primary and secondary sensory areas (Elston and Rosa, 1998; Elston et al., 2001; Jacobs et al., 2001; Gilman et al., 2017; Galakhova et al., 2022). A similar size increase in basal arborization along the caudal-rostral axis has been reported in elephants (Jacobs et al., 2011) and rodents (Benavides-Piccione et al., 2006; Elston et al., 2006), although with less pronounced regional differences (Mohan et al., 2015; Gilman et al., 2017). A cross-species comparison indicates that upper-layer neurons in frontotemporal regions of macaques and humans have a larger total dendritic length (on average 1.5-fold for macaques and 3-fold for humans) and greater branching complexity than homologous neurons in mice (Mohan et al., 2015; Gilman et al., 2017), whereas such differences are not observed in primary visual cortices (Gilman et al., 2017). When comparing mammals with large brains, the length of the basilar dendritic arborization in elephants is reported to be slightly longer in both frontal cortex (~7%) and occipital cortex (~3%) than in humans, despite a lower degree of branching in the former species (Jacobs et al., 2011).
Traditionally considered as a homogeneous cell population, there is increasing evidence for depth-dependent differences in the morpho-functional properties of L2/3 pyramidal cells in rodents (Brecht et al., 2003; Lübke et al., 2003; Staiger et al., 2015) and for the presence of neuronal subclasses distinguished by the expansion of their apical dendritic arborization, as observed in the rat medial prefrontal cortex (van Aerde and Feldmeyer, 2015). This morphological diversity of L2/3 pyramidal neurons appears to be even more pronounced in humans, with a significant increase in the extent of the horizontal field span of the apical tree, length of the basal arborization and mean radius of the cell body with increasing distance from the pia (Figure 3A) (Deitcher et al., 2017; Berg et al., 2021). In addition, recent RNA-sequencing studies have revealed the existence of two additional transcriptomically defined subtypes of pyramidal neurons in deep human L3, apparently absent in mice (Hodge et al., 2019; Berg et al., 2021).
The vast majority (70–95%) of excitatory synaptic inputs target the dendritic spines of neocortical pyramidal neurons (Nieuwenhuys, 1994). The elongated dendritic trees in multimodal and association cortices often correlates with an increase in spine density, particularly evident in frontal regions in primates (Elston et al., 2001; Jacobs et al., 2001). However, this trend does not hold true for all species. For examples, pyramidal neurons in the prefrontal cortex of the marmoset have fewer branches and spines than those in the temporal lobe (Elston et al., 2001), and the density of spines in the macaque prefrontal cortex does not exceed that observed in different regions of the mouse neocortex (Gilman et al., 2017). The length of dendritic spines (~1.3 μm combining neck and spine head) does not seem to significantly change with cortical size (Karbowski, 2014), despite a slight tendency for spines in the human temporal cortex to have longer necks (0.9 vs. 0.7 μm) and larger heads (0.6 vs. 0.4 μm2) compared to those in mice (Benavides-Piccione et al., 2002). Finally, in line with the idea that a dynamic balance between excitatory and inhibitory activities is a fundamental principle of cortical circuit function in physiological conditions, the ratio between excitatory (~70–90%) and inhibitory (~10–30%) synapses appears globally conserved in mammals, although laminar-dependent differences within and between species can be observed (for reviews DeFelipe et al., 2002; Karbowski, 2014).
4.3.2 Deep-layer pyramidal neurons in eutheriansInfragranular pyramidal neurons integrate inputs form virtually all neocortical layers thanks to their elongated apical dendrite and radiating basilar arborization. They in turn significantly influence cortical and subcortical operations through local connectivity and long-range output projections. L2/3 inputs to L5 cells are distributed along the dendritic tree, mainly on tuft branches, apical oblique and basal dendrites. Afferents from L4 mostly terminate on basal dendrites, which also receive axons from primary relay thalamic nuclei. Additionally, L5 pyramidal cells receive thalamocortical projections from higher-order thalamic nuclei on their apical tuft in L1 and basal dendrites (Weiler et al., 2008; Petreanu et al., 2009; Hooks et al., 2011). L5 ITs establish many reciprocal connexions and provide synaptic inputs to L5 ETs. Connexions from ETs to ITs are less numerous (Morishima and Kawaguchi, 2006; Brown and Hestrin, 2009; Kiritani et al., 2012); ET intracortical projections mainly contributing to inter-areal communications (Nelson et al., 2013; Ueta et al., 2013; Harris and Shepherd, 2015). Most of inputs to L6 ITs in sensory cortices originate from local deep-layer neurons, while L6 ETs are primarily innervated by axons from higher-order cortical areas (Zhang and Deschênes, 1998; Mercer et al., 2005; Feldmeyer, 2012; Vélez-Fort et al., 2014).
Although IT and ET neurons are distributed across L5 and L6, they display laminar-dependent projection patterns. For instance, corticostriatal neurons projecting to ipsilateral and/or contralateral striatum are found throughout L5 (Anderson et al., 2010; Pidoux et al., 2011a), while corticospinal neurons seem to be confined to the deeper part of L5 (Anderson et al., 2010; Suter et al., 2013), and corticothalamic neurons predominate in L6 (Bourassa and Deschênes, 1995). Corticothalamic neurons from sensory areas typically project back to their primary relay thalamic nuclei, but those located in the deeper part of L6 may also project to higher-order thalamic nuclei (Bourassa and Deschênes, 1995; Chevee et al., 2018).
The two subclasses of infragranular pyramidal neurons differ in their apical dendritic architecture, with great variability existing within each subpopulation in all species (for review, see Baker et al., 2018a). Traditionally, L5 ET neurons possess a more complex apical dendritic arborization with numerous branches and a crown-shaped tuft that unfolds close to the pial surface (Figure 3B), whereas the apical dendritic tuft of IT neurons is more restricted with fewer side branches (Hattox and Nelson, 2007; Ramaswamy and Markram, 2015; Kalmbach et al., 2021). Morphological heterogeneity is also present within L6; L6 ETs exhibit a relatively compact apical dendritic arborization predominantly terminating in narrow tufts in L4, while corticocortical L6 neurons extend an untufted or sparsely tufted apical dendrite, rarely extending beyond L4-L5 border. By contrast, the apical dendrite of the corticoclaustral IT neurons in L6 can reach the lower boundary of L1 (Katz, 1987; Zhang and Deschênes, 1997; Kumar and Ohana, 2008; Oberlaender et al., 2012; Yang et al., 2022). Consistent with inter-areal variations observed in superficial layers, infragranular pyramidal cells from higher processing areas possess a more elaborate basal dendritic arborization in the macaque (Elston and Rosa, 2000). Despite the difficulty of unambiguously identifying ET neurons in humans (based on their axonal projections), a transcriptomic cell class sharing multiple distinctive marker genes and morphological attributes with the murine ET neuron subtype (Tasic et al., 2016) has been identified in different regions of the human neocortex, despite a relative lower abundance as compared to monkeys and rodents (Hodge et al., 2019; Bakken et al., 2021; Kalmbach et al., 2021).
Certain subpopulations of ET neurons, with morphological features that deviate from the archetypal pyramidal neuron, are endemic to certain cortical areas and species. For instance, the corticospinal gigantopyramidal neuron (Betz cell in primates), with its very large cell body and extensive basilar dendrites, is exclusively found in the primary motor cortex of carnivores and primates (reviewed in Jacobs et al., 2018). The same applies to the large von Economo neuron, which is characterised by its spindle-shaped soma and thick, poorly branched apical and basal dendrites. Initially described in human anterior cingulate and frontoinsular cortices, they were first considered to be specifically human and identified as particularly prone to early loss and morphological alterations in various neuropsychiatric disorders (reviewed in Butti et al., 2013). Their existence, with similar cortical distributions and in fairly comparable numbers, was subsequently demonstrated in other large-brained species such as great apes, elephants and certain cetaceans, although their presence in non-primate mammals is still debated (Butti et al., 2013; Banovac et al., 2019).
4.3.3 Pyramidal neurons in marsupials and monotremesPyramidal neurons have been clearly identified in both marsupials and monotremes (Figure 3B), but a detailed analysis of their dendritic architecture and synaptic connectivity is still lacking (marsupials: Walsh and Ebner, 1970; monotremes: Dann and Buhl, 1995; Tyler et al., 1998; Elston et al., 1999; Hassiotis and Ashwell, 2003; Hassiotis et al., 2005; Jacobs et al., 2018).
Several key features of the eutherian pyramidal cell are retained in marsupials, including their presence throughout layers 2 to 6, an upward-projecting apical dendrite, elaborate basilar dendritic arborization, and recurrent excitatory synaptic connexions. Some variations, such as the more common bifurcation of apical dendrites into daughter branches, were however observed in wallabies, quokkas, and opossums, but not in dunnarts (Walsh and Ebner, 1970; Tyler et al., 1998; Jacobs et al., 2018). Our knowledge on neuronal classes in monotremes is still limited but the few existing studies suggest that pyramidal neurons in the short-beaked echidna represent a smaller proportion (35–50%) of the total population of cortical neurons compared to therian species. In addition, a substantial number of these pyramidal neurons (30–40%) display atypical attributes such as apical dendrites lacking a terminal bouquet or branching close to the soma, and poorly developed basal dendritic skirts. Monotreme pyramidal cells also appear to have a lower density of spines on apical and/or basal dendrites. However, the morphology of the different types of non-pyramidal neurons (spiny stellate cells and inhibitory interneurons) is very similar in monotremes, marsupials, and placentals. These observations led Hassiotis and colleagues to put forward the hypothesis that pyramidal and non-pyramidal neurons may have emerged as distinct morphological entities in the first mammals, while the entire set of typical pyramidal cell features would have appeared shortly after the split with the prototherian lineage, around 180 million years ago (Hassiotis and Ashwell, 2003; Hassiotis et al., 2005).
In summary, the morphology of eutherian pyramidal neurons appears more diverse than previously thought, even within a single cortical area of a given species (see, for example, Figure 3A). Furthermore, it is interesting to note that the early bifurcation of apical dendrites in marsupials and monotremes is an anatomical feature that is also commonly observed in some placental species, such as hedgehogs or elephants (Valverde and Facal-Valverde, 1986; Jacobs et al., 2011). Thus, the canonical and non-canonical aspects of pyramidal cells seem to have been relatively well preserved during cortical evolution; a better understanding
Comments (0)