PURPOSE The advent of new medical devices allows patients with asthma to self-monitor at home, providing a more complete picture of their disease than occasional in-person clinic visits. This raises a pertinent question: which devices and parameters perform best in exacerbation detection?
METHODS A total of 149 patients with asthma (90 children, 59 adults) participated in a 6-month observational study. Participants (or parents) regularly (daily for the first 2 weeks and weekly for the next 5.5 months, with increased frequency during exacerbations) performed self-examinations using 3 devices: an artificial intelligence (AI)-aided home stethoscope (providing wheezes, rhonchi, and coarse and fine crackles intensity; respiratory and heart rate; and inspiration-to-expiration ratio), a peripheral capillary oxygen saturation (SpO2) meter, and a peak expiratory flow (PEF) meter and filled out a health state survey. The resulting 6,029 examinations were evaluated by physicians for the presence of exacerbations. For each registered parameter, a machine learning model was trained, and the area under the receiver operating characteristic curve (AUC) was calculated to assess its utility in exacerbation detection.
RESULTS The best single-parameter discriminators of exacerbations were wheezes intensity for young children (AUC 84% [95% CI, 82%-85%]), rhonchi intensity for older children (AUC 81% [95% CI, 79%-84%]), and survey answers for adults (AUC 92% [95% CI, 89%-95%]). The greatest efficacy (in terms of AUC) was observed for a combination of several parameters.
CONCLUSIONS The AI-aided home stethoscope provides reliable information on asthma exacerbations. The parameters provided are effective for children, especially those younger than 5 years of age. The introduction of this tool to the health care system might enhance asthma exacerbation detection substantially and make remote monitoring of patients easier.
Key words:INTRODUCTIONAs defined in the Global Initiative for Asthma (GINA) annual report, asthma is a heterogeneous disease, usually characterized by chronic airway inflammation and a history of respiratory symptoms such as cough, wheezes, shortness of breath, and chest tightness, together with variable expiratory airflow limitation.1 It is the most common chronic disease of childhood and is a major source of childhood health burden worldwide, affecting 10% to 12% of children.2-4 It is also a prevalent respiratory disease among adults, affecting approximately 5% of men and 6.5% of women in the European Union.5
Asthma exacerbations represent a progressive deterioration of symptoms and lung function with respect to the patient’s usual status.1 Although asthma control can be reached in most cases within 1 year, data suggest that this is mostly achieved at the cost of maximizing drug dosages (68% and 76% of patients receiving salmeterol/fluticasone and fluticasone, respectively).6 Asthma exacerbations are associated with substantial health and economic effects including acute emergency department visits and occasional hospitalizations. Therefore, early diagnosis of an exacerbation is important for proper management and symptom relief. The identification and confirmation of an exacerbation is time sensitive, making efficient and effective home monitoring vital. Supplementary pulmonary function tests measuring peak expiratory flow (PEF) or forced expiratory volume in 1 second are available for home use. Whereas these tests are appropriate for use by adults and school children, they are not designed for use in children younger than 5 years of age.7,8
To achieve effective asthma management, patients should be given the necessary tools to allow them to recognize and respond to worsening asthma. Whereas GINA identifies continuous sounds (wheezes, rhonchi) as crucial for exacerbation detection,1 auscultatory changes have thus far been assessed mainly by doctors during face-to-face meetings with patients.
In this study, we investigated an artificial intelligence (AI)-aided home stethoscope, StethoMe (StethoMe sp. z o.o.), which enables the detection of pathologic auscultatory phenomena (including continuous sounds [wheezes and rhonchi] and transient sounds [coarse and fine crackles]) automatically at home as well as the effective measurement of other parameters such as heart rate (HR), respiratory rate (RR), and inspiration-to-expiration duration ratio (I/E). The aim of this study was to investigate which parameters are of crucial importance in exacerbation detection and monitoring of patients with asthma and to what extent a home AI-aided stethoscope can support this process, especially in children, for whom there is a lack of efficient tools. We assessed data for a 6-month monitoring period of patients with asthma. Examinations were performed at home using an AI-aided home stethoscope, PEF (when possible) and peripheral capillary oxygen saturation (SpO2) measurements, and additional survey information.
METHODSStudy DesignWe conducted an observational study of patients of various ages with asthma. We monitored asthma-related physiologic parameters (Table 1) at home for 6 months. Each patient (or parent) was required to perform health status (self-)examinations using a home stethoscope, peak flow meter, and pulse oximeter and indicate additional symptoms, including subjective breathing quality, and collect data as listed in Table 1, Figure 1, and Figure 2. The data were gathered with a dedicated smartphone application. Before the data collection process, each patient (or parent) was trained with respect to correct device usage and registration of all data in the provided mobile application. All information was sent to a platform accessed by physicians who analyzed the data to identify exacerbation occurrences.
Table 1.Tools Used and Related Data Collected by Participants at Home During Each Examination

 Figure 1.
Figure 1. Auscultation points on the chest according to age group: (A) <12 years of age, (B) >12 years of age.
 Figure 2.
Figure 2. Survey completed with a mobile application before each examination.
PEF = peak expiratory flow.
According to the research protocol, for the first 14 days, 1 examination (collection of a set of recordings from auscultation points on the chest performed ≥30 minutes after administration of asthma control drugs) per day was required, followed by ≥1 examination per week for the remaining duration of the program. When exacerbation or any alarming symptoms occurred, the participant was obliged to perform examinations twice a day. When determined by the physician assigned, participants were asked to perform additional examinations.
ParticipantsThe patient consent form and the approval from the Poznań University of Medical Sciences Bioethics Committee are shown in Supplemental Appendix 1 and Supplemental Appendix 2, respectively. Patients were recruited from the general Slavic population via advertisements and recruitment efforts conducted by medical centers. The inclusion criterion for children and adults was diagnosed asthma (primary or secondary care). In younger children, suspicion of asthma was also accepted. Any other comorbidities that might affect asthma assessment or influence the measured parameters constituted exclusion criteria. Each participant underwent individual evaluation, during which a physician assessed their eligibility for inclusion in the study. Neither season nor pollen activity were factors considered for selection; however, the majority of participants collected data during the autumn.
A total of 149 patients participated in the study. Among the 6,442 complete examinations, which included a total of 41,872 recordings, 6.4% did not meet quality criteria and were not analyzed further; exclusion was determined by StethoMe AI on the basis of inadequate quality of the majority of recordings for an examination. All remaining examinations were labeled by physicians, and 282 examinations from 54 patients were identified as moderate or severe exacerbations (Table 2).
Table 2.Patients and Examination Information
EquipmentWe used standard certified medical devices, including pulse oximeters (Accurate FS20P, Accurate FS20C [Hunan Accurate Bio-Medical Technology Co, Ltd]) and peak flow meters (Mini Wright Peak Flow and Low Range [Clement Clarke International]), to collect SpO2 and PEF measurements. Other parameters were measured with the European Conformity (CE)-certified StethoMe stethoscope, which records auscultatory sounds from standard chest points (Figure 1) and transfers sound files wirelessly to a dedicated mobile phone application. The recordings were automatically analyzed by the AI module, and results (pathologic auscultatory sound intensities, HR, RR, I/E) were displayed in the application. In addition, the user provided other health state information in a survey in the mobile telephone application (Figure 2).
We used StethoMe AI for automatic analysis of auscultatory recordings and aggregation of results for each examination (set of recordings from multiple auscultatory points on the chest). StethoMe AI has shown high effectiveness in respiratory sound recognition9-13; it was trained using >10,000 recordings of respiratory sounds (see Grzywalski et al9 for details) and is clinically validated and CE certified as a Class IIa medical device in Europe. This AI module is a specialized artificial neural network (NN) suitable for polyphonic sound event detection. It is composed of a dozen specialized layers of neurons, including many convolutional layers, which are effective at detecting local patterns in the signal, and several recurrent layers designed to capture long-time dependencies (eg, a patient’s breathing cycle and the associated recurrence of pathologic sounds). The network contains a total of 7.4 million trainable parameters. For output, the NN provides a matrix termed the probability raster. In this data structure, the columns represent time, discretized into 10-ms frames, and the rows indicate the probability of phenomena (ie, wheezes, rhonchi, coarse and fine crackles) detection changing over the frames. The probability values provided by the NN are thresholded to obtain Boolean values indicating the presence or absence of a phenomenon in each frame.
Sufficient quality of captured sounds was ensured by an inherent mechanism incorporated within the StethoMe AI. The quality assurance process relied on the following 2 primary factors: (1) the detection of the presence of breathing cycles, and (2) the absence of excessive background noise. Examination quality was then determined by evaluating the individual recordings. Quality assessment results were immediately available to patients on completion of the examination. In the case of rejection, users were encouraged to repeat auscultation for the low-quality points.
Reference StandardData were analyzed by physicians who assessed the asthma exacerbation level (none, mild, moderate, severe) for each examination. The assessment was carried out at the examination level rather than the recording level. A total of 17 physicians with different specializations were involved in this process (2 internal medicine specialists, 4 pulmonologists, 9 pediatricians, 5 allergologists, 4 family medicine specialists [some had a double specialization]), each assessing examinations performed by participants assigned exclusively to them. Each physician took into account all of the information generated by the participant (Table 1), listened to each auscultation recording, and analyzed the spectrogram (Supplemental Figure 1). Data from prior examinations were also available. These assessed examinations were considered the reference standard and were used to develop and evaluate the performance of machine learning classifiers.
Data AnalysisAll experiments and data analyses were carried out using Python 3.8 (Python Software Foundation) extended with the following packages: numpy version 1.19.5, scikit-learn version 1.0.2, scipy version 1.7.1, and pandas version 1.3.1. The raw data are accessible in Supplemental Appendix 3 in the form of a comma-separated values file.
We used the collected data to evaluate the effectiveness of different user-registered parameters (Table 1) for autonomous detection of asthma exacerbation. For this purpose, 12 feature sets were defined, each including patient age, gender, an identifier physician assessing the exacerbation level, and ≥1 user-registered parameters from Table 1. For each evaluated set, a random forest regressor (RFR) was fitted to the reference standard exacerbation levels mapped to a scale from 0 to 1.0 (none: 0; mild: 1/3; moderate: 2/3; severe: 1). Each RFR consisted of 100 decision trees trained using the squared error criterion and without constraining their maximum depth. For quantitative evaluation of the performance of the RFR, we rescaled the model’s prediction labels from the 4-point scale to binary values by thresholding at 0.5 (values >0.5 were mapped to 1, and values <0.5 were mapped to 0) so as to predict the existence of at least moderate exacerbation level, as identified by the physician’s labels. The model’s area under the receiver operating characteristic (ROC) curve (AUC) achieved on a held-out test set was used as a measure of the effectiveness of each considered feature set in the detection of asthma exacerbation.14-20 Each feature set was evaluated in a 10-fold cross-validation experiment, and each experiment was repeated 20 times to estimate the feature set’s mean AUC and 95% CIs. In each fold of the cross-validation process, the data set was categorized into a training set comprising 90% of patients. This training set was used to construct the RFR decision trees. In addition, a test set consisting of 10% of patients was created to evaluate the performance of the final model. Given that an individual’s data were never used for training and evaluation of the same RFR, the results show true model performance for patients not seen by the model.
We selected the RFR model as the predictor for exacerbation level on the basis of a series of preliminary experiments. These experiments involved testing various alternative models including decision trees; support vector regressors with linear, radial basis function, and sigmoid kernels; multilayer perceptrons; adaptive boosting (AdaBoost); gradient-boosting regressors; and their ensembles. After thorough evaluation, we selected the RFR primarily because of its consistent performance across different subsets of analyzed features and fast computation. In addition, we optimized the meta-parameters of the RFR model using an automated machine learning method. We found that the optimal forest only marginally outperformed the default forest when evaluated on the held-out test set. As a result, we decided to retain the default RFR metaparameters.
RESULTSFigure 3 shows the ROC curves obtained for the classifiers. To determine a standard performance measure, we calculated AUCs (Table 3 and Figure 4). Considering the ROC curves, the shapes for the different parameters and sets of parameters differed, which was reflected in the AUC values. For children, the ROCs with greatest curvature were obtained for the classifiers that used all provided input data (combination of all StethoMe AI analysis results, PEF, SpO2, and survey data) as well as those that used only data provided by the AI-aided stethoscope (4 pathologic sound intensities, HR, RR, and I/E) (shown in red and pink, respectively, in Figure 3), resulting in the greatest AUC values (younger children: 93.2% [95% CI, 92.1%-94.4%] and 93.0% [95% CI, 92.1%-93.9%], respectively; older children: 92.4% [95% CI, 90.9%-93.9%] and 92.4% [95% CI, 91.1%-93.7%]) (Table 3). For adults, the survey data performance (symptoms) was quantitatively similar to that of the curve corresponding to the use of all input data (AUCs 92.0% [95% CI, 89.4%-94.6%] and 93.7% [95% CI, 92.1%-95.3%], respectively), whereas the set of parameters provided by the AI-aided stethoscope (pink curve) was more flattened (AUC 81.0% [95% CI, 75.1%-86.8%]). In addition, all of the data depicted by single parameters (HR, RR, I/E, PEF, and peripheral capillary oxygen saturation) were relatively close to the diagonal of the plot (Figure 3), suggesting poor performance of these parameters in classification tasks for all age groups (AUCs close to or <70%). The most efficient single value parameters were continuous respiratory sounds (wheezes and rhonchi, orange curves). In this case, the AUC values were the lowest for adults (approximately 70%), whereas they reached approximately 80% for children.
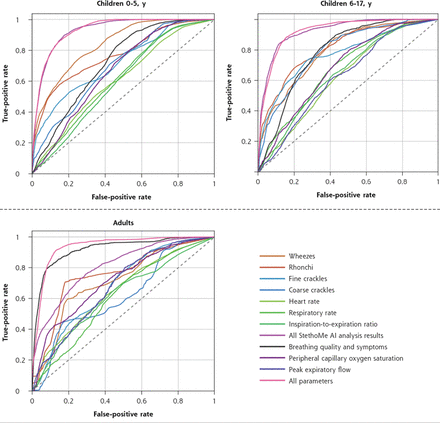
 Figure 3.
Figure 3. Receiver operating characteristic curves for classifiers based on different sets of home-collected data (physiologic parameters and survey data).
AI = artificial intelligence.
Note: Performance was measured on an independent validation set that included only patients not used during classifier training.
Table 3.Effectiveness of Different Sets of Home-Collected Data in Decision Support Systems Classifying Asthma Exacerbations Measured Using AUC
DISCUSSIONGiven that the diagnosis and monitoring of asthma is most challenging in children, especially younger children,21 this group was of paramount importance in this study, and we therefore discuss the findings for this group first. The Global Initiative for Asthma suggests that in children younger than 5 years of age, the evaluation of exacerbations should be based mainly on subjective assessment of the patient’s condition. Interestingly, Figures 3 and 4 clearly show that the subjective information noted by parents was not sufficient to confirm or exclude the appearance of an exacerbation (AUC 72.0% [95% CI, 70.1%-73.9%]) (Table 3). Our findings are consistent with prior research showing inconsistency in assessment between parents and doctors.22 Home monitoring of children is therefore strongly biased by the subjective assessment of lay persons (parents), and thus far there has been no tool to objectivize this. Our present results indicate that using a single-parameter approach, the determination of exacerbation is ambiguous, that is, PEF, SpO2, HR, RR, and I/E measurements appear to be relatively weak indicators of exacerbation in all groups of patients. Our findings are in line with the results of Muñoz-López23 and Brand et al,24 who suggest that during treatment, the PEF variation over time shows poor concordance with changes in other parameters of asthma severity. In addition, some authors suggest poor performance of HR and RR as determinants of acute asthma severity,25 which is confirmed by the present study. Constrained to single parameters, we found that continuous auscultatory sounds are more effective, with the AUC reaching approximately 80% for children.
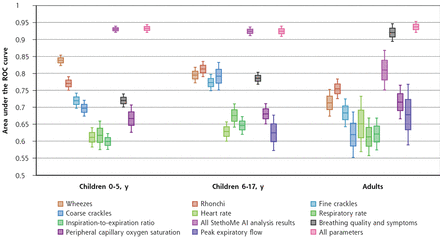
 Figure 4.
Figure 4. Results of the effectiveness (in terms of AUC) of different sets of home-collected data in decision support systems classifying asthma exacerbations.
AI = artificial intelligence; AUC = area under the ROC curve; ROC = receiver operating characteristic.
Note: The central line in each box denotes mean AUC, box edges extend to 1 SD from the mean, and fences (whiskers) denote 95% CI.
Only incorporation of several parameters into a single classifier appeared to be sufficient to detect exacerbations in children. The data provided by AI-aided stethoscopes (including survey), together with PEF and SpO2, performed the best in identifying asthma exacerbation in all groups; nevertheless in both children groups, equally high efficiency was obtained when considering the parameters provided by AI-aided stethoscope alone. This means that using AI-aided stethoscopes facilitates the detection of exacerbations with high effectiveness even in children younger than 5 years of age. Our present results strengthen the statements by Priftis et al26 and the Consensus Document of the Emilia-Romagna Asthma Study Group27 and are in line with the priorities for future research into asthma diagnostic tools given by the European asthma research innovation partnership.28
In contrast, in adults, the key to effective diagnosis of an exacerbation was patient reporting of disease-specific symptoms (Figures 3 and 4). Adding other parameters (AI-aided stethoscope data, PEF, and SpO2) only slightly improved performance. One might therefore state that adults can precisely describe their health status, and it can be difficult for parents when describing their children’s health status.
Regarding needs and recommendations for the future, it should be noted that asthma management is becoming much more patient specific.29 Therefore, AI-aided stethoscopes are a particularly useful tool that can be applied to optimize and improve patient-doctor collaboration using telemedicine solutions. It has been reported that telehealth programs have strong potential with regard to asthma-related health outcome improvement,30 increased access to care,27,28 and cost effectiveness.31,32 Currently, it is possible to send medical records via the internet and also to preanalyze them using AI modules. Still, there are no exact guidelines on how to objectively monitor aspects other than apparent asthma symptoms at home. As a result, establishing a definitive action plan is particularly challenging for younger children, owing to the limitations of conducting standard physiologic tests such as peak-flow measurements or spirometry. Our present results suggest that the use of AI-aided home stethoscopes might fill this gap by monitoring a broader set of parameters.
Strengths and LimitationsThe present results are based on large-scale data gathered from intended-use cases by certified medical devices, thus their reliability, especially considering home use, is much greater than that of a laboratory study with a limited number of participants and short-term monitoring. It is important to highlight that currently there are no definitive objective parameters or a specific set of objective parameters that can unequivocally confirm asthma exacerbation. The identification of asthma exacerbation relies on symptoms and indirect measures rather than concrete biomarkers. Thus, the reference standard we used was also established on the basis of the experience and subjective decisions of individual physicians. Nevertheless, this is fully consistent with the current clinical approach to asthma exacerbation detection. Finally, the present study focused solely on Slavic patients; however, there are no available data from GINA indicating any ethnic influence on exacerbation detection.
ConclusionsThe present results clearly show that a set of parameters (wheezes, rhonchi, coarse and fine crackles, HR, RR, I/E) measured by a device such as an AI-aided home stethoscope allows for the detection of exacerbations without the need for performing PEF measurements, which can be equivocal. In addition, in the case of younger children (aged <5 years), when introduced on a large scale, the analyzed home stethoscope appears to be a promising tool that might make asthma diagnosis more straightforward and substantially facilitate asthma monitoring.
FootnotesConflicts of interest: K.J., B.K.-K., A.Z, M.Ł., T. G., omasz Grzywalski, A.P., A.B., K.S., J.K., and H.H.-D. were paid for their work by the National Centre for Research and Development (grant no. POIR.01.01.01-00-0648/20). T.G., A.P., A.B., and K.S. are employees of StethoMe Sp. z o.o. J.K. and H.H.-D. are employees and shareholders of StethoMe Sp. z o.o. All other authors report none.
Read or post commentaries in response to this article.
Funding support: This work was supported by grant no. POIR.01.01.01-00-0648/20 from The National Centre for Research and Development (Poland).
Ethical and regulatory framework: This work was approved by the Poznań University of Medical Sciences Bioethics Committee (resolution no. 961/21) (Supplemental Appendix 2).
Received for publication March 31, 2023.Revision received June 23, 2023.Accepted for publication August 1, 2023.© 2023 Annals of Family Medicine, Inc.
留言 (0)