Objective. To integrate a Dynamic Collimation System (DCS) into a pencil beam scanning (PBS) proton therapy system and validate its dosimetric impact. Approach. Uncollimated and collimated treatment fields were developed for clinically relevant targets using an in-house treatment plan optimizer and an experimentally validated Monte Carlo model of the DCS and IBA dedicated nozzle (DN) system. The dose reduction induced by the DCS was quantified by calculating the mean dose in 10- and 30-mm two-dimensional rinds surrounding the target. A select number of plans were then used to experimentally validate the mechanical integration of the DCS and beam scanning controller system through measurements with the MatriXX-PT ionization chamber array and EBT3 film. Absolute doses were verified at the central axis at various depths using the IBA MatriXX-PT and PPC05 ionization chamber. Main results. Simulations demonstrated a maximum mean dose reduction of 12% for the 10 mm rind region and 45% for the 30 mm rind region when utilizing the DCS. Excellent agreement was observed between Monte Carlo simulations, EBT3 film, and MatriXX-PT measurements, with gamma pass rates exceeding 94.9% for all tested plans at the 3%/2 mm criterion. Absolute central axis doses showed an average verification difference of 1.4% between Monte Carlo and MatriXX-PT/PPC05 measurements. Significance. We have successfully dosimetrically validated the delivery of dynamically collimated proton therapy for clinically relevant delivery patterns and dose distributions with the DCS. Monte Carlo simulations were employed to assess dose reductions and treatment planning considerations associated with the DCS.
Pencil beam scanning (PBS) proton therapy is a form of radiotherapy that consists of magnetically scanning millimeter-wide pencil beams to deliver therapeutic radiation treatments to tumors in successive energy layers [1, 2]. In PBS, however, treatment quality in terms of lateral target conformity is often limited by the system's effective spot size at the patient surface [3–5]. To combat this issue, static apertures can be milled to conform to the projection of the largest energy layer in a given treatment to collimate the scanned field. While this does provide advantageous dosimetric benefits through the reduction of the effective spot size near the target edges, static collimators result in under-collimation of all other energy layers, an effect that is exacerbated for irregular shaped target volumes [6]. To address this, external dynamic collimation devices have gained interest in improving lateral conformity in PBS proton therapy [6–20]. These devices, categorized as per-field apertures, multileaf collimators, and sliding bar collimators, enable dynamically collimated proton therapy (DC-PT) [15].
One specific external collimation device, the Dynamic Collimation System (DCS), is being developed in conjunction with the IBA ProteusPlus system [13, 14, 20]. The Dynamic Collimation System (DCS) is a sliding-bar external collimation device that consists of four nickel trimmer blades that rapidly and independently move to intercept the scanning beam as it approaches the target edges. The DCS is equipped with an optional polyethylene range shifter to treat at depths below 4 cm. The current clinical DCS prototype utilizes trimmers that are 3 cm thick to fully attenuate 160 MeV protons and nickel to minimize neutron production [21]. The maximum energy was selected following the findings of Bues et al, who reported a loss of benefit with collimation at a depth of 17.5 cm [22]. The DCS is mounted on a telescoping snout that allows for varying trimmer-to-surface distances (TSD), defined as the distance between the bottom edge of the lowest collimating trimmer and the surface of the phantom.
Recent developments for this technology have consisted of the construction of a clinical prototype of the DCS [13] and a central axis alignment trimmer quality assurance device [23] as well as the development of a Monte Carlo model of the DCS mounted to the DN system [14]. More recently, an analytical algorithm has been developed to enable clinical treatment planning with the DCS [24–26]. While significant progress has been made in the development of the DCS, much of it has been confined to computational treatment planning studies and simplified experimental setups that do not fully represent clinical treatment scenarios.
The objective of this work is to provide an experimental basis of a complete collimated delivery from a clinically integrated DCS. To achieve this, the DCS control system will be integrated with the IBA beam scanning controller system, enabling the automated delivery of DC-PT with the DCS. Previous experimental studies have been limited to single beamlet irradiations or static trimmer configurations [8, 9, 12, 14, 27]. This work extends beyond those limitations by developing and simulating clinically relevant delivery patterns, parameterizing the expected dosimetric benefits of the DCS, and comparing the measured results with Monte Carlo simulations. In previous investigations, the impact of TSD on achievable penumbra has been evaluated for simple cubic fields [27]. However, this effect has yet to be addressed and experimentally verified for more clinically relevant dose distributions resulting from more complex delivery patterns. Additionally, this study explores the number of trimmer configurations requested per layer and considers the tradeoffs between dosimetric gains and delivery accuracy. By undertaking these simulation and experimental validation efforts, this research aims to bring DC-PT with the DCS closer to practical implementation, contributing to its potential clinical application.
2.1. Treatment planningA Monte Carlo-generated beamlet library and analytical plan creation techniques were employed to develop a set of uncollimated and collimated monoenergetic treatment fields consisting of one single energy layer. These fields were carefully designed to mimic clinically relevant delivery patterns that necessitate multiple trimmer positions per energy layer to achieve optimal treatment. The primary objective behind developing these fields was to facilitate the integration of the DCS control system with the IBA beam scanning controller. Additionally, these realistic target geometries served the purpose of offering valuable insights into the expected dose reductions in the normal tissue surrounding the target when utilizing the DCS system. The intention was to provide an experimental benchmark to healthy tissue sparing with the DCS.
In this study, we considered circular shapes with varying diameters of 3 cm, 5 cm, and 8 cm, as well as two kidney bean shapes of different sizes for dosimetric characterization in silico, however, experimental efforts in this work focused only on the large kidney bean shape. Figure 1 provides an illustration of these shapes. The selection of kidney bean shapes was inspired from the AAPM Task Group 53 (TG-53) [28], which provides guidelines for quality assurance procedures related to treatment planning systems, including MLC testing. Circular targets with varying diameters were chosen due to their simplicity, generalizability, and their resemblance to an energy layer used for treating a spherical target.
Figure 1. Illustration of target geometries evaluated in silico.
Download figure:
Standard image High-resolution image 2.1.1. Monte Carlo beamlet libraryThe Dynamic Collimation Monte Carlo (DCMC) package [29] was employed to simulate uncollimated and collimated beamlets, which were used to populate pencil beam libraries for the beamlet selection and weight optimization algorithm. The DCMC package is an open-source extension library based on TOPAS [30] that incorporates a beam model of the IBA Dedicated Nozzle (DN) system at the Miami Cancer Institute, along with a model of the trimmer components used to simulate the DCS system. Previous work has already validated this model against DCS-collimated beamlets, both in the presence and absence of an external polyethylene range shifter [14].
Non-range shifted (NRS) and range shifted (RS) beamlets were generated to treat targets at depths ranging from 2 cm to 22.5 cm. For simulations at a depth of 22.5 cm, the trimmer thickness was increased within the Monte Carlo model from 3 cm to 4 cm to ensure full attenuation of protons with energies greater than 160 MeV. Table 1 provides a summary of the treatment depths and beams considered for the monoenergetic treatment fields developed. The range shifter is required for treatment depths below 4 cm, which corresponds to the range of the lowest available beam energy at the Miami Cancer Institute (MCI) of 70 MeV. For depths beyond 4 cm, both RS and NRS plans were evaluated because RS beamlets used for treating at depths of 5 cm and 10 cm exhibit a smaller spot size at isocenter compared to their lower-energy NRS counterparts (see table 1,  for 5 cm depth). Thus, this study also serves as an evaluation of the utility of the range shifter for depths beyond 4 cm, providing additional guidance to end users regarding its benefits. Additionally, the effect of the TSD on the achievable dosimetric benefits was evaluated at a depth of 5 cm using RS- and NRS-based deliveries, considering TSD values of 5, 10, and 15 cm.
for 5 cm depth). Thus, this study also serves as an evaluation of the utility of the range shifter for depths beyond 4 cm, providing additional guidance to end users regarding its benefits. Additionally, the effect of the TSD on the achievable dosimetric benefits was evaluated at a depth of 5 cm using RS- and NRS-based deliveries, considering TSD values of 5, 10, and 15 cm.
Table 1. Summary of treatment depths evaluated in addition to the range shifter presence (RS = range shifted, NRS = non-range shifted), the beam energy, and spot size at isocenter for each respective depth.
Treatment depth (cm)RS or NRSBeam energy (MeV) (mm)2RS86.97.63RS94.87.05RSa109.36.2 NRSa78.36.610RS140.54.9 NRSa116.05.013.5RS159.74.315NRSa146.14.217.5NRS159.73.822.5NRS184.63.4
(mm)2RS86.97.63RS94.87.05RSa109.36.2 NRSa78.36.610RS140.54.9 NRSa116.05.013.5RS159.74.315NRSa146.14.217.5NRS159.73.822.5NRS184.63.4a Indicates depths for which measurements were performed.
The energy-specific beamlet libraries included an uncollimated beamlet and collimated beamlets with trimmer offsets of 1 and 2 mm for each trimmer, as shown in table 2. A minimum offset of 1 mm was selected based on the findings of Smith et al (2020) [31], who investigated the robustness of DC-PT with the DCS in terms of spot positioning, trimmer position, and mounting alignment uncertainties associated with the IBA universal nozzle system and concluded that implementing a minimum trimmer offset could improve the robustness in delivery with minimal impact on the high-dose conformity afforded by the DCS. The following assumptions were made within the beamlet library:
(1)
Beamlets can only experience collimation by a maximum of two trimmers.
(2)
Beamlets collimated by two trimmers simultaneously can only experience collimation by orthogonal trimmer pairs (e.g., any X trimmer with any Y trimmer, but not both X trimmers).
(3)
Symmetry is assumed between X and Y trimmers, allowing for beamlet kernels to be rotated and flipped to model collimation from an opposing trimmer (e.g., an X1-collimated beamlet can be flipped to model an X2-collimated beamlet).
Table 2. Table of unique trimmer offsets considered in beamlet library. 'Out' indicates the trimmer was placed out of field and did not interact with the beamlet, resulting in an uncollimated final configuration.
Configuration #123456789X offset (mm)1122OutOut12OutY offset (mm)121212OutOutOutTOPAS (Version 3.8.p1) Monte Carlo simulations were performed for the nine unique configurations (table 2) for each energy investigated. The dose to water was scored within a three-dimensional (3D) 1 mm isotropic dose grid embedded in a 40 × 40 × 40 cm3 water phantom, using 107 histories per configuration. The default TOPAS physics modules (g4em-standard_opt4, g4h-phy_QGSP_BIC_HP, g4decay, g4ion-binarycascade, g4h-elastic_HP, and g4stopping) were utilized for all simulations. Range cuts of 20 mm were placed on secondary electrons and gammas, while a 0.05 mm range cut was placed on secondary protons. All simulations were carried out using the computation assistance of the University of Wisconsin- Madison Center of High Throughput Computing (CHTC) cluster.
Following simulation of the X2- and Y2-collimated beamlets, two-dimensional (2D) dose distributions at the Bragg depth were analytically modified to account for collimation by all possible trimmer combinations (X1, X2, Y1, Y2), resulting in a total of 32 unique trimmed beamlets and one uncollimated beamlet for each energy. A set of the X2- and Y2-collimated beamlets at an energy of 78.3 MeV are illustrated in figure 2. These 2D beamlet libraries were then utilized to create and optimize uncollimated and collimated treatment plans for the targets in figure 1.
Figure 2. Simulated 78.3 MeV beamlet dose profiles for the trimmer configurations listed in table 2. Profiles are relative and displayed at the depth of the Bragg peak at 5 cm in water. DCS trimmers are overlaid in gray.
Download figure:
Standard image High-resolution image 2.1.2. Beamlet selection processUncollimated and collimated single energy treatment plans were created using a two-step process: beamlet selection and weight optimization. First, candidate beamlets were selected for a given target geometry based on a pre-defined dosimetric criteria,  Following the selection of candidate beamlets, a linear least squares weight optimizer was used to provide a uniform dose of 5 Gy to the target. To evaluate the dosimetric benefits of the DCS and enable equivalent comparisons between uncollimated and collimated treatments, the following 2D dose-area based criteria were maintained throughout the planning process:
Following the selection of candidate beamlets, a linear least squares weight optimizer was used to provide a uniform dose of 5 Gy to the target. To evaluate the dosimetric benefits of the DCS and enable equivalent comparisons between uncollimated and collimated treatments, the following 2D dose-area based criteria were maintained throughout the planning process:
(1)
Doses to 98% and 2% (e.g., D98% and D2%) of the target areas must be within 3% of the prescription dose (4.85 and 5.15 Gy) for all plans.
(2)
Uncollimated and collimated D98% and D2% values must be within 1% of each other.
The beamlet selection algorithm proposed by Hyer et al was utilized to select beamlets for both uncollimated and collimated treatment fields, where beamlets are analytically shifted to model off-axis spots according to a pre-determined fixed spot map [7, 8]. For this work, all treatment plans utilized an in-plane spot spacing of 2.5 mm, with the exception the small kidney bean which utilized a 1 mm spot spacing because of the smaller size of the target. For each spot position, the dose ratio ( ) for each beamlet was calculated with respect to a pre-defined simplified conformity function,
) for each beamlet was calculated with respect to a pre-defined simplified conformity function, 
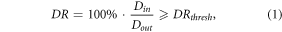
where  is the total integrated beamlet dose inside the target and
is the total integrated beamlet dose inside the target and  is the total integrated beamlet dose outside of the target. The
is the total integrated beamlet dose outside of the target. The  parameter largely influences the number of beamlets placed outside of the target for subsequent weight optimization. Following the beamlet selection algorithm, a weight optimization is carried out using a linear least squares optimizer developed by Flynn et al (2007) [32]. This optimizer considers point- and volume-based (or for this work, area-based) dosimetric penalties to define an optimization objective function. In general, the parameters used to define these objective functions were tuned to yield identical target coverage between the uncollimated and collimated cases.
parameter largely influences the number of beamlets placed outside of the target for subsequent weight optimization. Following the beamlet selection algorithm, a weight optimization is carried out using a linear least squares optimizer developed by Flynn et al (2007) [32]. This optimizer considers point- and volume-based (or for this work, area-based) dosimetric penalties to define an optimization objective function. In general, the parameters used to define these objective functions were tuned to yield identical target coverage between the uncollimated and collimated cases.
For the creation of collimated fields, the number of collimated beamlets utilized in the plan, or level of collimation, must be decided. The level of collimation is dictated by a pre-defined threshold denoted as Tcoll
, which influences the number of collimated spots selected in each irradiation pattern. The influence of the  parameter is illustrated in figure 3 for uncollimated and DCS-collimated plans that utilized two differing levels of collimation (
parameter is illustrated in figure 3 for uncollimated and DCS-collimated plans that utilized two differing levels of collimation ( = 40% and 70%) a depth of 15 cm. The influence of Tcoll
is illustrated in the difference between figures 3(b) and (c). The mathematical definition of this parameter and its influence on DCS-collimated dose distributions is further discussed in the appendix. In general, however, increasing values of Tcoll
increases the number of trimmed beamlets in the irradiation patterns. The influence of the level of collimation on deliverability was assessed experimentally at two levels for each treatment depth evaluated.
= 40% and 70%) a depth of 15 cm. The influence of Tcoll
is illustrated in the difference between figures 3(b) and (c). The mathematical definition of this parameter and its influence on DCS-collimated dose distributions is further discussed in the appendix. In general, however, increasing values of Tcoll
increases the number of trimmed beamlets in the irradiation patterns. The influence of the level of collimation on deliverability was assessed experimentally at two levels for each treatment depth evaluated.
Figure 3. Spot maps for (a) uncollimated and (b and c) collimated large kidney bean treatments placed at a depth of 15 cm for collimation thresholds ( ) of (b) 40% (b) and (c) 70%. The uncollimated plan utilized a
) of (b) 40% (b) and (c) 70%. The uncollimated plan utilized a  value of 30% and both collimated plans utilized a
value of 30% and both collimated plans utilized a  value of 16%.
value of 16%.
Download figure:
Standard image High-resolution image 2.2. Mechanical integrationTo enable the delivery of DCS-collimated treatment fields, modifications were made to the pencil layer definition (PLD) file, which is responsible for describing the irradiation patterns. The PLD file is converted into machine files for execution by the IBA Beam Delivery Control Unit (BDCU). The PLD file consists of layer blocks and elements, where layer blocks define the irradiation pattern for a specific energy layer, and elements contain information about the spot map and meterset weights (MU) for each spot. To incorporate trimmer positions, the PLD file was modified by adding the trimmer positions to the end of each element line. These trimmer positions were then transferred to a customized BDCU equipped with two I/O boards responsible for communicating the trimmer positions to the DCS control system. The DCS control system, in turn, provides feedback to the BDCU, enabling it to determine when the trimmers are correctly positioned. Once the trimmers are in place, the BDCU continues its normal operation and delivers the spot according to the specified MU.
2.3. Experimental dosimetryExperimental validation was conducted to verify the accuracy and deliverability of DCS-collimated treatment plans. The validation focused on large kidney bean plans targeting depths of 5, 10, and 15 cm without the range shifter (NRS), as well as 5 cm with the range shifter (RS). To ensure accurate measurements, planar 2D dose distributions were obtained in the plateau region proximal to the Bragg peak to avoid dosimetric uncertainties near the peak. The measurements were performed in water using the MatriXX-PT (IBA Dosimetry, Schwarzenbruck, Germany) [34, 35] ionization chamber array and EBT3 radiochromic film (Ashland Specialty Ingredients, Bridgewater, NJ, USA) within in the DigiPhant PT scanning water tank. For 10 and 15 cm depth plans, measurements were taken at depths of 6 and 11 cm, respectively. For the 5 cm depth plans, measurements were performed at a depth of 4 cm, the shallowest possible measurement plane within the water tank. Central axis dose measurements were also conducted at the reference depths of 6 and 11 cm using the IBA PPC05 reference class ionization chamber. However, it was not feasible to perform PPC05 measurements at the 4 cm depth due to geometrical constraints.
The primary objective of these measurements was to validate the automated deliverability of DCS-collimated treatment plans in comparison to the simulated dose distributions and absolute dosimetry modeling achieved through Monte Carlo treatment planning. The accuracy of the Monte Carlo code's dose calibration, expressed as Gy/proton, was verified by comparing it to the measured dose in Gy/MU. This comparison allowed for the determination of a Monte Carlo conversion factor in protons/MU under the reference conditions specified by IAEA TRS-398 [36]. These energy-specific conversion factors were then used to relate optimized weight in terms of protons to MU within the PLD delivery file.
Following the completion of the beamlet weight optimization, all plan parameters, including energy, spot positions, trimmer positions (if applicable), and number of MUs, were written as PLD files for delivery using a modified version of the IBA BDCU. The MatriXX measurements employed a dual irradiation technique, involving two measurements with a 3.8 mm shift to generate a dose distribution with a native resolution half that of the chamber-to-chamber spacing. The EBT3 films were scanned using an Epson® Expression 10000XL (Epson America, Inc., Long Beach, CA) flatbed scanner at 300 dpi, which corresponds to a spatial resolution of 0.085 mm. All dose distributions were interpolated to a spatial resolution of 0.5 mm, and an optimized rigid registration was used to align the measured and simulated dose distributions through translations and rotations. The average central axis doses within the lateral dimensions of a PPC05 chamber were derived from the Monte Carlo and MatriXX-PT dose distributions. To assess agreement, absolute gamma analysis was performed between Monte Carlo and MatriXX-PT dose distributions, while relative gamma analysis was conducted between Monte Carlo and film dose to avoid LET saturation effects [37, 38].
While these measurements were performed with clinical tools, such as the MatriXX-PT, the absence of a spread-out Bragg peak distribution makes absolute dose verification more complicated in regions of dose gradients as a function of depth. Despite the measurements being performed proximal to the Bragg peak, the shallowest measurements performed at a depth of 4 cm were still in a relatively high-dose gradient region (5 %/mm) near the pristine Bragg peak at a depth of 5 cm. Because of this, a distance to agreement (DTA) in the depth direction was considered to account for any uncertainties associated with depth positioning. Planar 2D gamma analyses were carried out using criteria of 3%/3 mm, 3%/2 mm, and 2%/2 mm to evaluate the expected agreement range, however, the 3%/3 mm criterion is widely adopted clinically for PBS proton therapy [35, 39–41]. A dose threshold of 10% was used in all gamma analyses to compute the pass rate, following the recommendations of AAPM Task Group 218 for photon-based intensity modulated radiation therapy quality assurance (QA) [42]. There are currently no official recommendations for gamma dose thresholds for patient specific QA in proton therapy.
3.1. Treatment planningThe uncollimated treatment fields were verified to have D98% and D2% values within 1.8% and 0.8% of the prescription dose, respectively, across all target geometries. In the case of collimated fields, the D98% and D2% values were verified to be within 1.5% and 0.8% respectively. Additionally, the differences in D98% and D2% values between the uncollimated and collimated plans were within 0.4% and 0.1% respectively. To achieve this agreement,  values of approximately 30% and 16% were required for the uncollimated and collimated plans respectively. The lower
values of approximately 30% and 16% were required for the uncollimated and collimated plans respectively. The lower  value for collimated plans indicates that a larger number of beamlets were placed outside the target geometry compared to the uncollimated case. This is a logical result, additional beamlets were necessary to achieve the desired target coverage due to the sharper penumbra of the collimated beamlets.
value for collimated plans indicates that a larger number of beamlets were placed outside the target geometry compared to the uncollimated case. This is a logical result, additional beamlets were necessary to achieve the desired target coverage due to the sharper penumbra of the collimated beamlets.
To benchmark and evaluate the protection of normal tissues achieved by the DCS, we analyzed the mean dose (D50%) to 2D peripheral rind regions surrounding the target areas with widths of 10 mm and 30 mm. These doses were studied in relation to treatment depth and the presence of a range shifter. Figure 4 illustrates the mean doses to the examined regions for both uncollimated and collimated plans, considering cases that were either range shifted (RS) or non-range shifted (NRS). Additionally, figure 5 displays the dose reductions induced by collimation in the RS and NRS scenarios for the evaluated regions and targets. The results presented in figures 4 and 5 are averaged across the circle and kidney bean targets evaluated in figure 1, where the error bars correspond to the type A variation across all targets.
Figure 4. Plots of the mean dose, as a percentage of the prescription dose, as a function of depth for 10 (left column) and 30 mm (right column) rinds surrounding the targets for the NRS (top row) and RS (bottom row) plans evaluated in silico. Error bars correspond to the type A variation in mean doses across the circle and kidney bean targets evaluated.
Download figure:
Standard image High-resolution imageFigure 5. Plots of the reduction in mean dose, as a percentage of the prescription dose, as a function of depth for 10 (left) and 30 mm (right) rinds surrounding the targets for the NRS and RS plans evaluated in silico. Error bars correspond to the type A variation in the mean dose reductions across the circle and kidney bean targets evaluated.
Download figure:
Standard image High-resolution imageIn general, the variations in target-specific dose reductions were more pronounced in the RS cases compared to the NRS cases. For both examined regions, the NRS plans exhibited a linearly decreasing dose reduction trend, while the RS cases demonstrated a non-linear behavior. In the NRS plans, the maximum simulated dose reductions for the 10- and 30-mm rinds were 11.6%  0.9% and 44.8%
0.9% and 44.8%  2.1%, respectively, at a depth of 5 cm. These reductions linearly decreased at rates of 0.58% and 2.08% per cm in water, respectively. On the other hand, the RS plans showed lower simulated dose reductions compared to the NRS plans, with reductions of up to 6.9%
2.1%, respectively, at a depth of 5 cm. These reductions linearly decreased at rates of 0.58% and 2.08% per cm in water, respectively. On the other hand, the RS plans showed lower simulated dose reductions compared to the NRS plans, with reductions of up to 6.9%  1.0% and 34.8%
1.0% and 34.8%  1.4% at a depth of 5 cm for the 10- and 30-mm rinds, respectively. The theoretical depths at which the polynomial fits of the dose reduction data reach zero indicate the point at which the benefits of collimation are no longer present. For the 30 mm rind, these benefits are lost at depths of 17.5 cm and 22.5 cm in water for the RS and NRS cases, respectively. The presented results were obtained using
1.4% at a depth of 5 cm for the 10- and 30-mm rinds, respectively. The theoretical depths at which the polynomial fits of the dose reduction data reach zero indicate the point at which the benefits of collimation are no longer present. For the 30 mm rind, these benefits are lost at depths of 17.5 cm and 22.5 cm in water for the RS and NRS cases, respectively. The presented results were obtained using
Global News and Health Forum
Comentarios (0)