Antibiotic resistance is one of the most critical matters confronting the clinical and industrial sectors, as well as the environment and social development. Antimicrobials are used excessively or unrestrictedly in humans and animals, which is really the leading cause of antibiotic resistance.[1]Acinetobacter baumannii, a non-fermenting member of the Moraxellaceae family, is the prominent bacterium that is responsible for hospital acquired diseases because it possesses various resistance mechanisms, such as the production of beta-lactamases, porin channel loss, reduced membrane permeability, modified antibiotic binding positions, and overexpression of efflux pumps[2] [Figure 1].
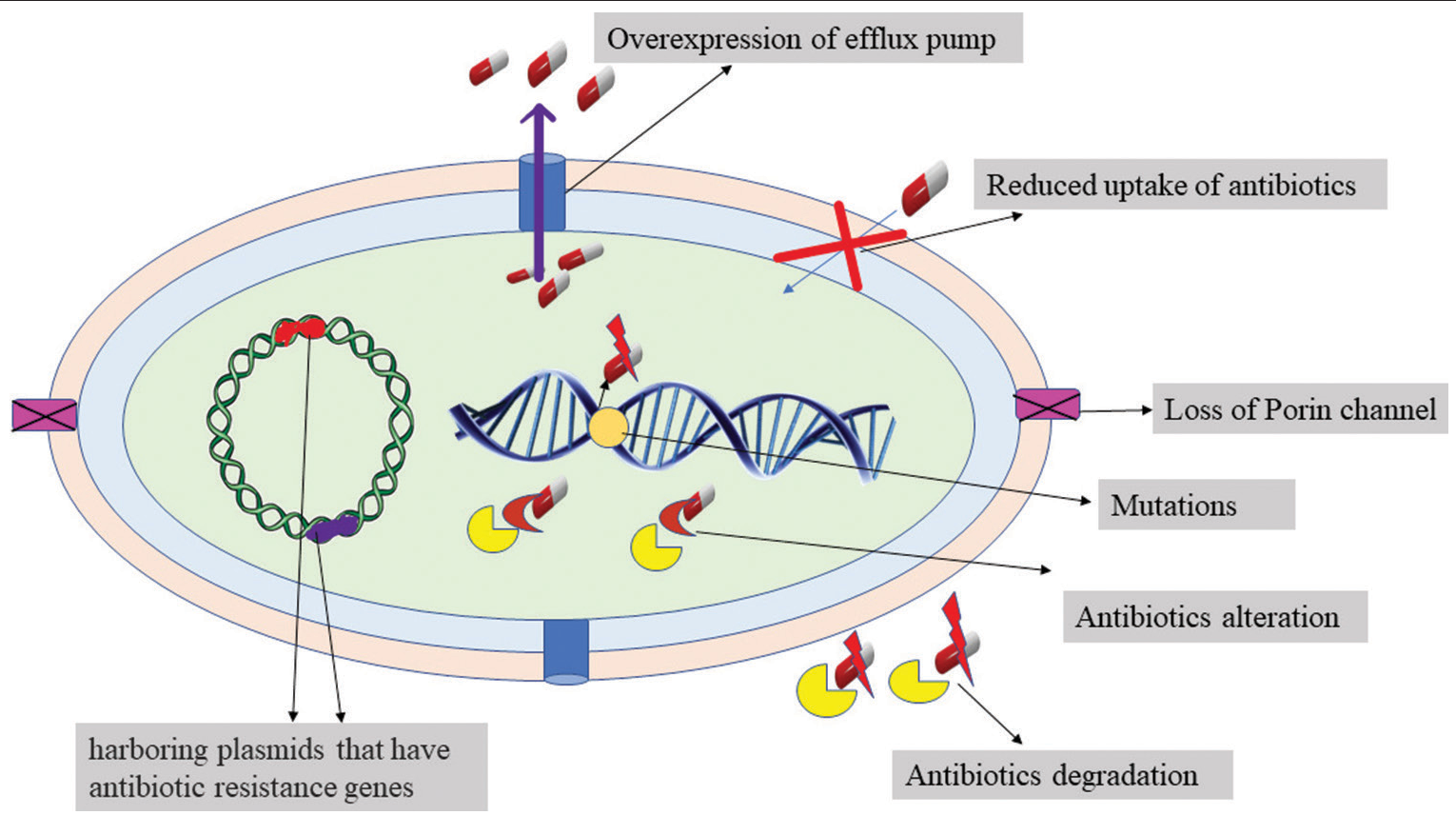
Figure 1:: Resistance mechanism of Bacteria.
Export to PPT
A. baumannii may be found in the soil and water, as well as on the skin of healthy individuals.[3] Patients who are on ventilators, have devices such as enteral feeding tubes and catheters, have open wounds postoperatively, are in intensive care units (ICUs), are immunocompromised, unconscious, have a history of taking certain antibiotics for prolonged period of time, or have protracted hospital stays are at risk for getting infection from this pathogen.[4]A. baumannii can also have the ability to use ethanol as a carbon source and survive desiccation.[5]
Multidrug resistance (MDR) A. baumannii infections are a significant concern in those patients who are hospitalized in the ICU.[6] It causes bloodstream infections, skin and soft-tissue infections, ventilator-associated pneumonia, urinary tract infections, meningitis, and endocarditis.[7] Inappropriate management and restricted therapeutic alternatives are responsible for poor outcomes and these infections are linked to high fatality rates, particularly among ICU patients.[8]
Carbapenems such as doripenem, imipenem, ertapenem, and meropenem are antibiotics used as the last option to treat diseases caused by MDR A. baumannii.[9] Carbapenem-resistant isolates, on the other hand, are appearing at an alarming rate through producing carbapenamase enzymes (Metallo-lactamases and Oxacillinases) that possess the ability to hydrolyze these antibiotics.[9-11]
Most existing treatment options, including carbapenems or combination antibiotic therapy, are futile because of A. baumannii immense adaptive capability to withstand the harsh environment and acquiring and disseminating antibiotic resistant genes through intrinsic or horizontal gene transfer. A. baumannii is the primary pathogen on the World Health Organization’s priority list for novel antibiotic research.[11-14]
Cross-infection between patients and inter/intraspecies transfer of resistance components is more likely when infection control methods are breached.[15] Furthermore, A. baumannii has a substantial capability to produce biofilms on a different type of surfaces, which not only boosts A. baumannii’s potential for nosocomial dissemination but also enhances antibiotic resilience and pathogenicity[16] [Figure 2].[2]
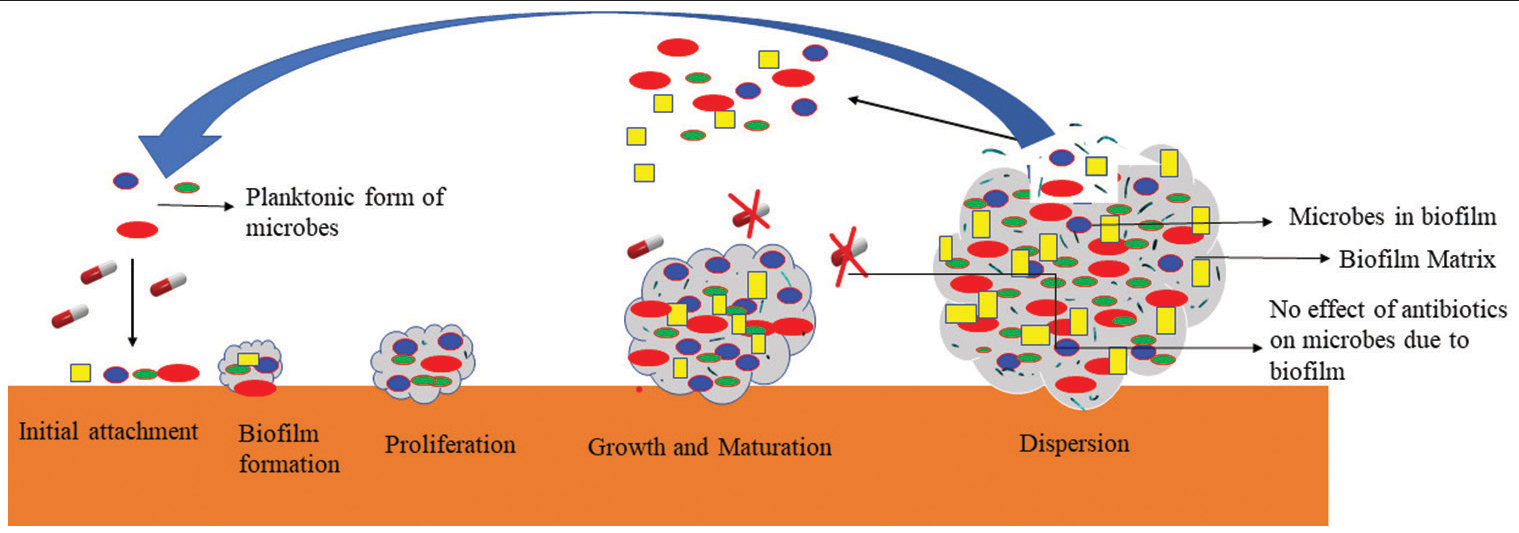
Figure 2:: Biofilm Formation.
Export to PPT
Colistin is currently thought to be the only medicine that has the potential to treat infections spurred by carbapenem-resistant A. baumannii;[11,17,18] Nevertheless, strains resistant to this antibiotic are also being identified.[19] Therefore, innovative new methods and drugs for treating A. baumannii infection are urgently required.
For many years, humanity has suffered the effects of a plethora of infection and disease-causing microorganisms. Extensive research projects have been carried out to analyze the usefulness of the plant extracts and active compounds in combating the problem of antimicrobial resistance in bacteria.[20] Plant extracts, which are complex combinations of main chemicals and their secondary metabolites, may have synergistic effects with conventional antibiotics.[21] Natural products have several advantages over synthetic antimicrobial chemicals, including fewer side effects, improved patient tolerance, low cost, wide acceptability due to traditional usage, renewability, and superior biodegradability.[22]
In various research studies, the following plant extracts and their essential oils are being used to combat A. baumannii infections: [Table 1]
Table 1:: Plant extracts and essential oils against Acinetobacter baumannii.
 Cinnamomum zeylanicum (CINNAMON)
Cinnamomum zeylanicum (CINNAMON)
Cinnamon has been utilized since 2000 BC in Ancient Egypt, when it was highly valued. It was used by doctors in mediaeval times to alleviate arthritis, coughing, and sore throats.[23] Cinnamon is mostly made up of essential oils and compounds such as cinnamic acid, cinnamaldehyde, and cinnamate.[24] Cinnamon has an anticancer, anti-inflammatory, antimicrobial, anti-diabetic, cardiovascular-disease-lowering, and lipid lowering capabilities.[25]
Cinnamomum verum is a tiny, evergreen Lauraceae family tree which is often recognized as a “real cinnamon tree” and “Ceylon cinnamon tree.” The bark of cinnamon trees, such as Cinnamomum cassia, C. verum, and other species, is used to make cinnamon.[26,27] This tree’s botanical name, C. zeylanicum, originates from “Ceylon,” the historic name for Sri Lanka.[27]C. cassia, popularly known as “Chinese cinnamon,” is an evergreen tree that is native to South China.[28]
The main components of Cinnamon and its essential oils are cinnamaldehyde, caryophyllene, eugenol, and linalool.[29] Cinnamon also contains essential components such as cinnamyl acetate, procyanidin-A and methyl cinnamate.[30] Essential oils and their secondary metabolites have been utilized as bactericides, insecticides, antiseptic, and fungicides since the middle ages. According to Morais et al., 2019 these components are often employed in the food and pharmaceutical sectors, as well as in cosmetics and medical devices manufacturing industries due to their diverse qualities.[31]
Cinnamon essential oil has antibacterial activity against antibiotic resistant pathogens such as methicillin resistant Staphylococcus aureus, A. baumannii, Pseudomonas aeruginosa, and vancomycin resistant Enterococcus faecium.[32] Essential oils have the capacity to limit bacterial multiplication and can minimize the required active concentration of antibiotics due to their synergistic effect. “Synergy” is defined as “when the combined impact is greater than the sum of the individual effect in combination treatment.” Combined effect, that is equal to the sum of all the individual impacts, has an additive effect.
Guerra et al., 2012 demonstrated that essential oils from C. zeylanicum and Citrus limon combined with amikacin had a synergistic impact against A. baumannii strains.[33,34] Essential oils are typically extracted using steam distillation or mechanical expression whereas plant extracts are commonly extracted using acetone, ethanol, or hexane.[35] Water condensate is separated from essential oil during distillation procedure through gravity, releasing a very little amount of volatile fluid. Essential oils may be able to advance from being traditional agents to being the commonly utilized therapy in the modern medical domain in the future[22] [Figure 3].
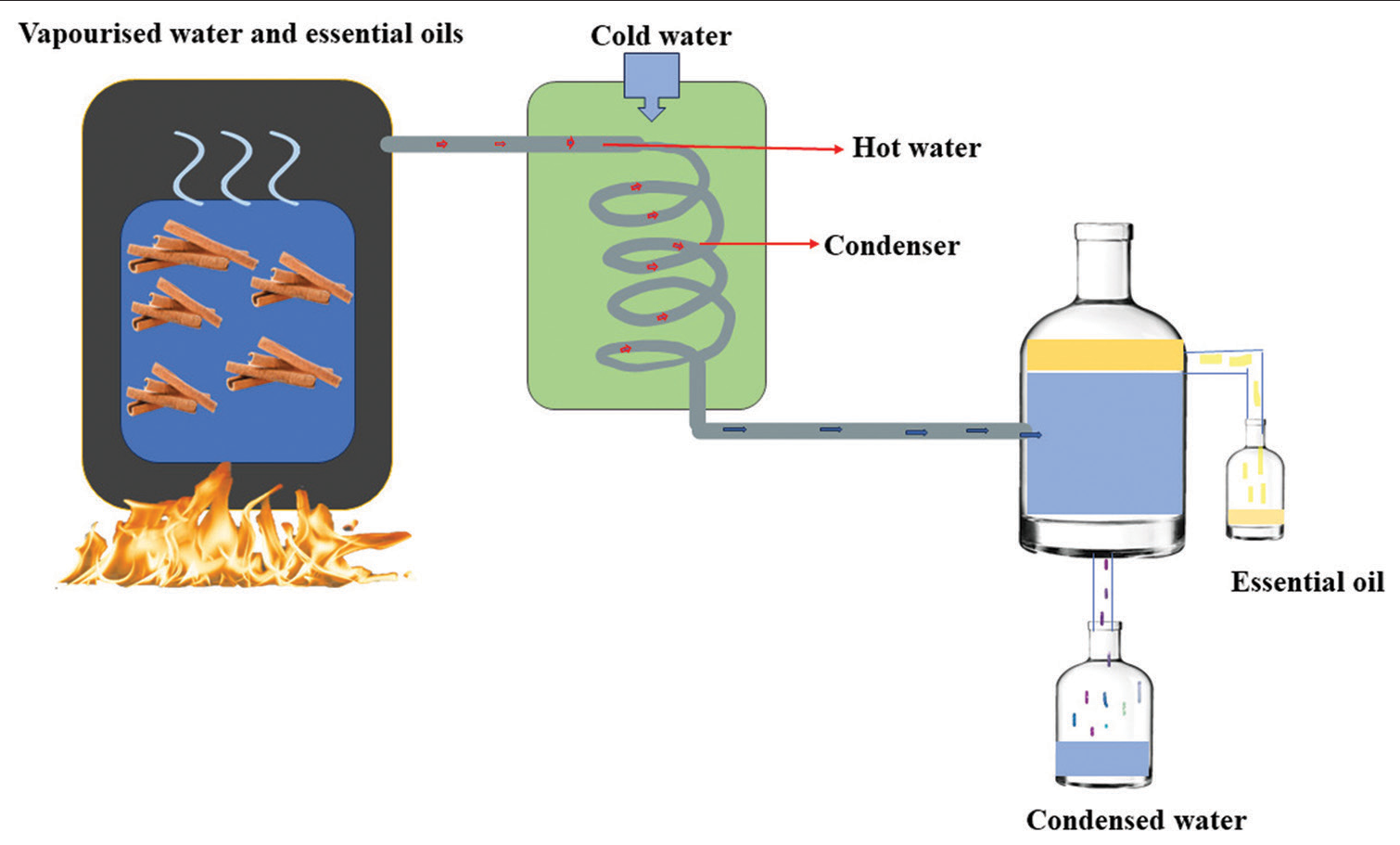
Figure 3:: Distillation process of Cinnamomum zeylanicum (Cinnamon).
Export to PPT
According to Karumathil et al., 2018 transcinnamaldehyde in combination with antibiotics enhanced A. baumannii susceptibility to lactam antibiotics such as penicillin, ampicillin, amoxicillin, piperacillin, methicillin, meropenem, and aztreonam.[36]
Sienkiewicz et al., 2014 assessed in their research study that all A. baumannii isolates obtained from medical devices and the environment with a degree of resistance, have shown to be inhibited by the essential oils.[34]
Thirapanmethee et al. 2021 demonstrated in their study that cinnamaldehyde displayed significant antimicrobial activity against all clinical strains in dose dependent approach, and it had no negative impact on the susceptibility of A. baumannii to any of the antimicrobials examined. Cinnamaldehyde had very low minimum inhibitory concentration (MIC) of 0.01–0.04% (v/v), and it had showed a minimum bactericidal concentration (MBC) that were equivalent to the MICs against most MDR A. baumannii strains. After treatment with cinnamaldehyde, the bacterial cells continued to elongate and cell separation was interrupted.[37]
Cinnamaldehyde’s antibacterial effect has lately been researched and a mechanism such as interaction with the cell membrane and fast suppression of energy metabolism has been proposed. Because of its hydrophobicity, it can infiltrate and disrupt the lipid bilayer of the cell membrane causing greater permeability to protons and the evacuation of essential chemicals and ions from bacterial cells, eventually leading to lysis of bacterial cells.
Mohamed et al. (2018) showed in their research study that low concentrations of cinnamaldehyde had excellent antibacterial and antibiofilm action against A. baumannii clinical strains that produce robust biofilms, with MIC and MBC of 0.875 and 1.75 mg/mL, respectively. Hence, they suggested that it might be utilized to treat biofilm-related clinical issues caused by this pathogen.[38]
Cinnamaldehyde decreases quorum sensing (QS) signaling at a specific concentration, which leads to a reduction of extracellular polymer production, which may also explain its powerful antibiofilm effect.[39]
Sabir and Sidiq demonstrated that when cinnamon nanoemulsions are combined with either kanamycin or gentamycin, the antibacterial activity of both antibiotics is significantly increased compared to when they are used alone. As a result, a combination like this might be utilized to combat MDR A. baumannii.[40]
Zingiber officinale roscoe (GINGER)Ginger (Z. officinale Roscoe) is a popular spice from the Zingiberaceae family that is grown all over the world, but particularly in Asian nations.[41] Ginger’s rhizome contains a unique homologous set of molecules known as Gingerols, which are the main phenolic plant’s secondary metabolites, responsible for the spice’s distinct flavor and health benefits.[42] Research projects have been undertaken widely over the last two decades to uncover bioactive components and the therapeutic efficiency of ginger.[43] Ginger’s antibacterial, anti-inflammatory, blood pressure-lowering, cholesterol-lowering, antiplatelet aggregation, chemo preventive, antioxidant, and hypoglycemic characteristics are all possible health advantages.[44]
According to Wang et al. (2010) four known component of ginger, 6-dehydrogingerdione, 6-shogaol, 10-gingerol, and 6-gingerol have been shown to have antimicrobial properties against extensively drug resistant A. baumannii (XDRAB). Their findings suggest that ginger compounds have antioxidant properties that can aid in antibacterial action and therefore could be used to treat XDRAB infections.[45]
Al Meani et al. (2020) in their research study observed that the effects of ginger volatile oil on carbapenamase production and carbapenamase activity were both positive. When used with the checkerboard approach with meropenem, ginger volatile oil had a synergistic impact against A. baumannii.[46]
Coriander sativum (CORIANDER)Coriander (C. sativum L.) belongs to the Apiaceae family and is used worldwide as medicinal herb with nutritional and therapeutic properties. The antibacterial, anticancer, antioxidant, antidiabetic, free radical, and antimutagenic properties of coriander extracts and essential oils have been demonstrated.[47] Although all parts of the coriander plant are edible, their flavors and applications may differ. The aerial parts of C. sativum have been the subject of most reported findings on antioxidant activity.[48]
Microdilution susceptibility tests and checkerboard assays were used to examine coriander oil’s antibacterial activity and synergistic interaction by Duarte et al. (2012) and antibiotics such as chloramphenicol, tetracycline, piperacillin ciprofloxacin, gentamicin, and cefoperazone were found to have synergistic or additive action with coriander oil against A. baumannii.[49]
Alves et al. (2016) evaluated the effectiveness of linalool on planktonic cells and biofilm of A. baumannii on different substrates. They also examined its effect on adherence and QS and they found that linalool had the greatest bactericidal activity of all the chemicals tested, with MICs ranging between 2 and 8 μL/mL. Linalool also inhibited the production of A. baumannii biofilms, dispersed existing biofilms, altered A. baumannii adherence to substrates, and hindered the quorum-sensing system.[50]
Aegle marmelos (BAEL)A. marmelos (Family Rutaceae), also called as “Bael” in Bengal and “Vilvam” in Tamil, is found in arid woods across India and is also farmed.[51] The blooms are greenish white, the leaves are trifoliate, the bark is yellowish brown, and the fruits are green with orange brown tasty sticky pulps. Asthma, emphysema, diarrhea, dysentery, dyspepsia, stomach discomfort, seminal weakness, uropathy, vomiting, diabetes, snake bite, seafood poisoning, and certain antiviral activity are all treated with extracts from various portions of the plant.[52]
Bael or A. marmelos contains steroid, tannin, terpenoids, phenol, glycoside, coumarin, alkaloids, and quinones, when combined with imipenem, Bael is particularly effective against A. baumannii strains (ATCC19606 which was carbapenem susceptible, and RS 307 which was resistant to carbapenem). However, it has a lower inhibitory impact when used alone. Antibiotics alone had a modest inhibition zone, but when combined with herbal extracts, they had a wider inhibition zone.[53]
Actinidia deliciosa (KIWI)Tiwari et al. (2017) discovered the anti-biofilm activity in aqueous extracts of A. deliciosa (Kiwi) and Syzygium aromaticum (clove) against A. baumannii.[54,55] Sanquinarine (alkaloid) as well as hydroxyflavone (flavonoid) are found in A. deliciosa extract. The anti-biofilm action of these extracts on A. baumannii extracellular matrix (ECM) revealed that it lowers the levels of exopolysaccharides, proteins, and extracellular deoxyribose nucleic acid in the ECM.
Origanum vulgare (OREGANO)Oregano (family-Lamiaceae) is a perennial plant which is native to the Mediterranean and Euro/Irano-Siberian Mountain ranges. Dried Oregano leaves are commonly utilized as a culinary herb in Italian cuisine, but they are also included in traditional medicine to cure indigestion, colds, and upset stomach due to the high carvacrol and thymol content naturally present in its essential oil.[56]
Microdilution (Resazurin Microtiter Assay), Time kill test, and Disk diffusion test were used to evaluate the antibacterial potential of O. vulgare L. essential oil (OVeo) to encounter carbapenem-resistant microorganisms by Vasconcelos et al. (2019) and found that O. vulgare essential oil has significant antibacterial action against MDR A. baumannii. They discovered that OVeo exhibited MIC of 0.015% (v/v) for A. baumannii.[57]
Another research work was done by Amaral et al. (2020), in which they evaluated the combination of Oregano essential oil (OEO) and polymyxin against MDR A. baumannii and found that carvacrol (71.0%) was the most common component of OEO, followed by –caryophyllene (4.0%), γ-terpinene (4.5%), p-cymene (3.5%), and thymol (3.0%). With MICs ranging from 1.75 to 3.50 mg/mL, OEO showed antibacterial activity against all A. baumannii-MDR strains. Flow cytometry revealed that the OEO promotes bacterial cell membrane instability and rupture, resulting in the death of A. baumannii cells. A checkerboard experiment revealed a synergic relationship between OEO and polymyxin B. When used together, OEO resulted in a 16-fold decrease in polymyxin B MIC. The findings suggest that using OEO alone or in combination with polymyxin B to treat A. baumannii MDR infections is a potential option.[58]
Eucalyptus camaldulensis (RED RIVER GUM)E. camaldulensis (red river gum) is a species of the Eucalyptus genus, which belongs to the Myrtaceae family, and includes over 900 species. E. camaldulensis essential oils and extracts are found to be most active against pathogens when they were compared with other species of genus Eucalyptus.
Knezevic et al. (2016) demonstrated that terpinen-4-ol, spatulenol, p-cimene, 1, 8-cineole, cryptone, and β-pinene were the most prominent components in E. camaldulensis essential oils. The MICs for E. camaldulensis essential oils were found to be in the range of 0.5–2 μL/mL. Time-kill curves for E. camaldulensis essential oils with polymyxin B lowered bacterial count under detection limit which was incredibly fast, that is, after 6 hrs of incubation, confirming the synergistic interaction.[59] In most cases, the studied essential oils had a synergistic antibacterial effect when they are used with traditional antibiotics such as polymyxin, ciprofloxacin, gentamicin, and in some situations, they even resensitized MDR A. baumannii isolates.
CONCLUSIONAntibiotic resistance in pathogenic microorganisms has sparked research attempts to find new medicines and produce efficient derivatives of already existing antibiotics because of the rapid growth of antibiotic resistance in pathogenic microbes, particularly to multiple prescribed drugs. However, no new antibiotics are being developed. Hence, plant extracts can be utilized for the benefit of human health as an alternative to therapeutic drugs and as an antibacterial supplement in health-care services that could cut costs while being safe. Yet, further research is needed, such as in vivo toxicity studies and clinical trials, to evaluate the efficacy of plant extracts.
留言 (0)