In inflammatory bowel disease (IBD), chronic inflammation leads to unfavorable clinical outcomes and increases the risk of developing colorectal neoplasm (CRN); thereby highlighting the importance of endoscopically evaluating disease activity as well as detecting and characterizing CRN in patients with IBD. With recent advances in image-enhanced endoscopic (IEE) technologies, especially virtual chromoendoscopy (VCE) platforms, this review discusses state-of-the-art IEE techniques and their applicability in assessing disease activity and surveillance colonoscopy in patients with IBD. Among various IEE, VCE demonstrated the capacity to identify quiescent disease activity. And endoscopic remission defined by the new scoring system using VCE platform better predicted clinical outcomes, which may benefit the tailoring of therapeutic strategies in patients with IBD. High-definition dye-chromoendoscopy (HD-DCE) is numerically superior to high-definition white light endoscopy (HD-WLE) in detecting CRN in IBD; however, discrepancy is observed in the statistical significance. VCE showed comparable performance in detecting dysplasia to HD-WLE or DCE and potential for optical diagnosis to differentiate neoplastic from nonneoplastic lesions during surveillance colonoscopy. Applying these novel advanced IEE technologies would provide opportunities for personalized medicine in IBD and optimal treatment of CRN in patients with IBD.
Keywords: Chromoendoscopy; Colorectal neoplasms; Image-enhanced endoscopy; Inflammatory bowel diseases; Virtual chromoendoscopy
INTRODUCTION Endoscopic examination is crucial for managing inflammatory bowel disease (IBD) to assess disease activity and detect neoplastic lesions. In IBD, mucosal inflammation is associated with unfavorable long-term outcomes including complications, flares, surgeries, and increased risk of bowel damage. Endoscopic healing is recommended as a long-term treatment target based on the selecting therapeutic targets in IBD II recommendation.1,2 Therefore, meticulous evaluation of disease activity and treatment response with endoscopy is becoming increasingly important to optimize therapeutic strategies. Additionally, patients with long-standing ulcerative colitis (UC) and colonic Crohn's disease (CD) have an increased risk of developing colorectal neoplasia (CRN) compared with the general population.3,4 However, detecting and characterizing CRN in IBD remain difficult because CRN frequently presents as flat or subtle morphologies that can be easily missed, and inflamed or regenerative mucosal changes prevent the identification of CRN in patients with IBD. In the last decade, endoscopic technologies have progressed and various image-enhanced endoscopy (IEE) techniques, including the recently developed advanced virtual chromoendoscopy (VCE), have enabled a more comprehensive and accurate evaluation of mucosal inflammation and detection of dysplasia. In this review, we provide an overview of the application of IEE in the assessment of disease activity as well as identification and characterization of CRN in patients with IBD. IMAGE-ENHANCED ENDOSCOPY Dye-chromoendoscopy Dye-chromoendoscopy (DCE) refers to endoscopic techniques that use a contrast dye (indigo carmine) or an absorptive agent (methylene blue or crystal violet) to enhance contrast and mucosal visualization. This enables endoscopists to delineate abnormalities such as morphological changes, lesion borders, surface pit patterns, or size of suspicious lesions.5 Indigo carmine is a widely used contrast dye in DCE. It is not absorbed by the mucosal gland but pools in the pits and innominate grooves, highlighting the pit pattern and mucosal structure, such as irregular mucosal depression, abrupt cutting, or swelling of the fusion folds. Adverse reactions such as bradycardia, hypertension, nausea, vomiting, and rarely, bronchospasm and hives may occur following indigo carmine administration. However, these reactions occur with intravenous administration, and almost no side effects have been observed when a small amount is used for DCE. Methylene blue and crystal violet are used in the absorptive method to stain epithelial cells. Thus, the area surrounding the pit is stained, whereas the pit remains unstained and appears white, enabling accurate visualization of the pit pattern. Methylene blue also differentiates mucosal lesions from normal mucosa based on the degree of staining uptake. Normal mucosa exhibits a diffuse homogeneous dark blue appearance following the methylene blue administration, whereas abnormal mucosal lesions with inflammation or dysplastic changes appear light blue or pink as unstained or heterogeneously stained. This allows the detection of CRN or determination of the extent of inflammation in patients with IBD. Methylene blue is safe for use in humans. Although concerns have been raised regarding the possibility of oxidative damage to DNA owing to photosensitization by white light endoscopy (WLE) after absorption by cells, these concerns have not been clinically proven. There is no evidence that the risk of cancer is increased in patients undergoing DCE using methylene blue. According to the “SURFACE” guidelines, which describe the process of performing DCE in patients with UC, DCE can be performed as follows.5,6 (1) Strict patient selection: DCE is ideal for patients with clinical remission and mucosal healing but avoided in patients with active disease, if possible. (2) Unmask the mucosal surface: excellent bowel preparation is essential, and mucus or residual fluid in the colon must be removed during insertion. (3) Reduce peristaltic waves: antispasmodics can be administered, if necessary, to reduce blind spots due to spasms or haustral folds. (4) Full-length staining of the colon: pan-chromoendoscopy is recommended over focal staining. (5) Augmented detection with dyes: before the procedure, 0.1% to 0.5% indigo carmine, 0.05% to 0.5% methylene blue, or 0.05% to 0.2% crystal violet dye solution is needed and usually about 80 to 100 mL dye solution is used per procedure. To prepare 0.1% indigo carmine solution, 5 mL of 0.4% indigo carmine is diluted with 15 mL of water. To achieve a final concentration of 0.1%, 5 mL of 1% methylene blue is diluted with 45 mL water.7 Different concentrations of dyes can be utilized depending on the purpose of the examination; for instance, a lower concentration is used for detection, whereas a higher concentration is used for characterizing the lesions. After cecal intubation, the colonic mucosa is meticulously inspected, and the lumen is dyed in a segmental fashion (every 20–30 cm) by withdrawing the colonoscope in a spiral fashion using a dye spray catheter or pump jet. It is important to use appropriate pressure while applying the dye using a catheter, as bleeding due to excessive injection pressure hinders the inspection of suspicious lesions. Direct injection of indigo carmine via a biopsy valve using a syringe can reduce the injection pressure and prevent bleeding. Additionally, it is crucial to apply a minimal amount of dye to prevent dye accumulation in depressed areas. After waiting for a few seconds for the dye to pool onto the mucosal surface, excess pools of indigo carmine are suctioned, and the mucosa is scrutinized. Indigo carmine staining persists for a few minutes and subsequently vanishes owing to dilution. Methylene blue requires approximately 60 seconds for absorption and the lesion can be examined for up to 20 minutes once stable staining occurs. (6) Crypt architecture analysis: pit pattern classification should be performed for all suspicious lesions. Types I and II indicate nonneoplastic lesions, whereas types III and V indicate neoplastic lesions, including carcinomas. (7) Endoscopic targeted biopsy: suspicious lesions with mucosal alterations should be biopsied, especially circumscribed lesions with stained types III–V pit patterns. Virtual chromoendoscopy VCE is widely used in endoscopic units to enhance the details of mucosal and vascular patterns without dye application. VCE includes various optical technologies such as narrow-band imaging (NBI; Olympus), iSCAN (Pentax), flexible imaging color enhancement (FICE; Fujinon), and blue laser light/linked color imaging (BLI/LCI; Fujifilm). Unlike a direct image acquired using standard WLE, which is obtained as a red-green-blue (RGB) image using the full visible wavelength range (400–700 nm), in NBI, shorter wavelengths of light between 415 nm and 540 nm (a narrow band of blue and green light) passing through an optical filter are absorbed by hemoglobin. Thus, the blood vessels appear dark, and the contrast in the mucosal layer is increased. This enables the assessment of precious mucosa and vascular patterns.8 Moreover, iSCAN, FICE, and BLI/LCI recreate virtual images using digital post-processing software.9,10 iSCAN has three modes set by the endoscopist for each condition using a touch screen: surface enhancement (SE), tone enhancement (TE), and contrast enhancement (CE). SE mode facilitates lesion detection by controlling illumination intensity. CE mode facilitates the identification and demarcation of lesions by digitally adding blue color to the relatively darker areas. In TE mode, the frequencies of RGB color are altered and combined with images in new color schemes. This enhances the visualization of the mucosal structure, vascular patterns, and subtle color changes, and helps characterize the lesions.10,11 Unlike iSCAN, which enhances per-pixel luminosity using white light, the recently developed iSCAN-optical enhancement (iSCAN-OE) increases overall transmittance of the hemoglobin absorption spectrum using an optical filter. FICE enables the visualization of tissue characteristics and vessels through signal processing, which extracts spectral images of specific wavelengths from white light. However, FICE poses difficulties in providing high-contrast images of microvessels under white light, which led to the development of BLI/LCI.12 The BLI system comprises a light source and a processor that creates high-contrast images by enhancing blue-violet light while reducing white light components. Although LCI utilizes the same illumination spectrum as BLI, it increases color contrast, resulting in a reddish color becoming redder and a whitish color becoming whiter; thereby maintaining natural tones. Therefore, LCI facilitates the detection of lesions and inflammation. Table 1 summarizes the mechanisms, strengths, and weaknesses of the available IEE techniques. ENDOSCOPIC EVALUATION FOR DISEASE ACTIVITY Endoscopic healing is a clinical parameter for predicting favorable outcomes and treatment targets for optimized medical therapy. However, some discrepancies exist between endoscopic and histological activity. Persistent histological inflammation is frequently observed in patients with endoscopic healing, which suggests the limitations of endoscopic evaluation of disease activity in patients with IBD.13,14 The limitations of endoscopic evaluation in capturing disease activity in patients with IBD may be attributed to two factors. First, current endoscopic scoring systems were developed using standard definition (SD)-WLE, which may not be sufficiently sensitive to detect subtle patchy changes in mucosal inflammation. Second, the current definition of endoscopic healing remains unclear. The lower end of the endoscopic inflammation assessment score was used to define endoscopic healing, which may have led to an inaccurate reflection of the actual state of mucosal inflammation. Therefore, several advanced IEE techniques have recently been adopted to accurately assess disease activity of IBD. Kudo et al.15 evaluated the performance of the NBI system to predict histological inflammation. They differentiated mucosal vascular pattern (MVP) into normal or distorted under conventional colonoscopy as well as clear or obscure under the NBI system. Comparing MVP to the histologic findings for inflammation, obscure MVP examined using NBI showed a significantly higher association with acute inflammatory cell infiltrates (26% vs. 0%, p=0.0001), goblet cell depletion (32% vs. 5%, p=0.0006), and basal plasmacytosis (2% vs. 21%, p=0.006) than clear MVP.17 Additionally, the shape of the capillary vessels with magnified NBI in patients with UC in remission correlated with the histologic findings, such that the blood vessels shaped like vines (BV-V) demonstrated higher pathological activity than that of honeycomb-like blood vessels (BV-H).16 Blood vessels shaped like bare branches (BV-BB) significantly predicted relapse at 12 months (odds ratio [OR], 14.2; 95% confidence interval [CI], 3.3–60.9]. However, because blue light with a wavelength of approximately 415 nm is mostly absorbed by hemoglobin, the NBI system poses some challenges in assessing vascular patterns in patients with moderate or severe disease activity accompanying intramucosal hemorrhage.10,15 In a randomized controlled trial (RCT), Neumann et al.17 compared disease activity and extent assessed using VCE, especially iSCAN, with those assessed using high-definition (HD)-WLE based on histological results in UC patients with mild or inactive activity. iSCAN showed a higher endoscopic prediction of inflammatory extent (92.31% vs. 48.71%, p=0.0009) and activity (89.74% vs. 53.85%, p=0.066) than that of HD-WLE. Iacucci et al.18 designed an endoscopic scoring system with iSCAN, which was combined with the scores for mucosal and vascular patterns. This scoring system showed a good correlation with Mayo endoscopic subscore [MES] (rs=0.86; 95% CI, 0.79–0.91; pppp19 Based on these results, the Paddington international virtual chromoendoscopy score (PICaSSO) was developed using iSCAN to redefine mucosal (elongated crypts, scars, microerosions, erosions, and ulcerations) and vascular changes (spare vessel, a vessel with dilatation, and intramucosal or luminal bleeding) from 0 to 15 to assess inflammation (Table 2).20-22 Endoscopic remission, defined as a score 22 Additionally, endoscopic remission evaluated by PICaSSO alone using iSCAN showed comparable predictive ability for certain clinical outcomes, such as hospitalization, colectomy, and medication change with a combination of endoscopic and histologic remission at follow-up over 12 months.23 In contrast, ulcerative colitis endoscopic index of severity (UCEIS) with histologic remission was more favorable for predicting certain clinical outcomes than UCEIS alone. Although PICaSSO was initially developed with the iSCAN platform, it had a good correlation with RHI and NHI when used with NBI (ρ=0.83; 95% CI, 0.751–0.902 and ρ=0.79; 95% CI, 0.678–0.87) or BLI/LCI (ρ=0.65; 95% CI, 0.482–0.781 and ρ=0.63; 95% CI, 0.472–0.754)/(ρ=0.65; 95% CI, 0.486–0.775 and ρ=0.63; 95% CI, 0.486–0.754) platforms, respectively.24 Additionally, the accuracies for predicting histologic remission of PICaSSO with NBI or BLI/LCI was comparable with the current scoring system, such as MES, UCEIS. Recent advances in artificial intelligence have led to the development of a novel VCE-convolutional neural network (CNN) model that can assess endoscopic remission and predict histologic remission as well as the risk of disease flare.25 The PICaSSO multicenter study used 1,090 endoscopic videos of 283 patients with UC for training, validation, and testing. Endoscopic activity was graded using UCEIS during HD-WLE, and PICaSSO during iSCAN 1–3. This CNN system detected endoscopic remission (PICaSSO≤3) with a sensitivity of 79%, specificity of 95%, and area under the receiver operator curve (AUROC) of 0.94, as well as predicting histological remission with an accuracy of 80% to 85%. To evaluate the diagnostic capability of LCI in detecting mucosal inflammation in UC, Uchiyama et al.26 categorized LCI patterns into three categories: A, no redness; B, redness with visible vessels; and C, redness without visible vessels. The LCI index had a good correlation with the histopathological Matts score and better predicted non-relapsed rates within 30 months after LCI examination (p=0.0055) than MES (p=0.0632). A retrospective Japanese study demonstrated that LCI could better identify histological mucosal inflammation (defined by GS 2–3) by detecting redness in the colonic mucosa, and better predict relapse in UC than WLE.27 These findings suggest that LCI can be a useful tool for detecting mucosal inflammation in patients with IBD. However, in a recent meta-analysis of 12 studies that compared the correlation between endoscopy (MES, UCEIS, PICaSSO) and histological disease activity scores (GS, NHI, RHI, NYMS) across SD-WLE, HD-WLE, and VCE in patients with UC, endoscopic activity scores demonstrated a strong correlation with histologic scores regardless of the endoscopic platform.28 However, subgroup analysis revealed that histologic remission was more accurately predicted by VCE than by WLE. Considering the results of current studies, VCE could help identify histologic remission or predict clinical outcomes in patients in an endoscopically quiescent state. PICaSSO demonstrated its potential as a reliable and accurate scoring system for endoscopic assessment using various VCE platforms in patients with IBD. Table 3 summarizes representative studies evaluating disease activity in IBD using IEE.15-19,22-27 ENDOSCOPIC EVALUATION FOR THE DETECTION OF CRN Patients with UC and colonic CD are at an increased risk of developing CRN, including carcinoma.3,4 However, chronic inflammation and a wide range of morphologic features of CRN, such as subtler and flat lesions, hamper accurate detection and treatment of CRN.29 To enhance the identification of CRN, the Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendations (SCENIC) guidelines recommends using HD-WLE or SD-DCE rather than SD-WLE alone, and suggested HD-DCE for surveillance colonoscopy in patients with IBD.29 With recent advances in resolution and virtual modalities, various IEE techniques, including DCE, NBI, iSCAN, and FICE, were studied to determine whether they can detect CRN in patients with IBD. Several studies compared CRN detection rates between HD-DCE and HD-WLE. A randomized trial by Alexandersson et al.30 demonstrated that the CRN detection rate of HD-DCE was more significant than that of HD-WLE alone (11% vs. 5%, p=0.03) and superiority of HD-DCE was also confirmed in the RCT by Wan et al.31 However, a multicenter prospective randomized trial by Yang et al.32 that enrolled 210 patients with long-standing UC reported that although the detection rate was numerically higher in the HD-DCE group, HD-DCE was not significantly superior to HD-WLE in detecting CRN (5.6% vs. 3.9%, p=0.749). Recent meta-analyses reported that the CRN detection performance of DCE was better than that of SD-WLE alone, whereas the CRN detection performance of DCE was comparable with that of HD-WLE, especially in the subgroup analysis confined to RCTs.33,34 When comparing NBI to conventional WLE, including HD-WLE, for detecting CRN in patients with long-standing UC, several studies have reported that NBI does not improve the detection of CRN. In a randomized crossover study that included 42 patients with UC, the CRN detection rate of NBI was comparable with that of conventional colonoscopy (19% vs. 17%, p=0.71).35 Although more suspicious lesions were observed (52 vs. 28, p=0.03) and more targeted biopsies were performed (148 vs. 85) during NBI than conventional WLE, this did not result in an actual increase in the detection rate of CRN. Another crossover RCT that included 25 patients with UC reported a comparable detection rate of CRN between NBI and HD-WLE (82% vs. 73%, p=1.00).36 However, this study found that nonneoplastic lesions were detected more frequently using NBI than HD-WLE, suggesting that NBI may have limited accuracy in differentiating neoplastic and nonneoplastic mucosa. Furthermore, HD-NBI was compared with HD-DCE for detecting CRN using targeted biopsy in two RCTs. In a crossover RCT that enrolled 60 patients with colonic IBD, HD-NBI demonstrated a similar detection rate for intraepithelial neoplasia (20% vs. 18.3%, p=0.43).37 Although withdrawal time was significantly reduced with HD-NBI than that with HD-DCE (15.74 vs. 26.87, pp=0.20). Another RCT that enrolled 131 patients with UC also showed that the CRN detection rate of HD-NBI was comparable to that of HD-DCE (21.5% vs. 21.2%, p=0.96); however, total procedure time was significantly shorter with HD-NBI than that with HD-DCE (25.0 vs. 32.5, p38 CRN detection by iSCAN was also compared with that of HD-WLE or DCE. Iacucci et al.39 evaluated CRN detection using iSCAN, HD-WLE, and HD-DCE in 270 patients with long-standing inactive IBD for surveillance colonoscopy. In this randomized non-inferiority trial, the CRN detection rate of iSCAN was not inferior to that of HD-DCE. The CRN detection rate of HD-WLE was not inferior to that of iSCAN or HD-DCE (18.9% vs. 11.1% vs. 17.8%, p=0.91), indicating that HD-WLE alone was adequate for detecting CRN in this study. Recently, Kandiah et al.40 demonstrated that the CRN detection rates with targeted biopsy were not significantly different between iSCAN and HD-WLE in an RCT that enrolled 188 patients with long-standing IBD (14.9% vs. 24.2%, p=0.14). Additionally, only one biopsy among the 6,751 non-targeted biopsies confirmed CRN, which suggested that targeted biopsy with iSCAN or HD-WLE is a good strategy for surveillance colonoscopy in patients with IBD. Recent meta-analyses have reported that CRN detection using VCE was comparable to that of DCE or HD-WLE.41,42 In a meta-analysis of 11 RCTs comparing VCE, including AF, FICE, iSCAN, and NBI with DCE and SD or HD-WLE in detecting CRN, the CRN detection of VCE was equivalent to that of DCE or HD-WLE in a per-patient analysis.41 In contrast, the CRN detection of VCE was inferior to that of HD-WLE and comparable to that of DCE in a per-dysplasia analysis.41 In another meta-analysis that assessed the effectiveness of different IEE techniques (SD or HD-WLE, DCE, AFI, and VCE including NBI, iSCAN, and FICE) for identifying CRN in patients with IBD, the CRN detection of DCE was better than that of SD-WLE.42 DCE showed significantly equivalent efficacy in identifying patients with CRN and the total number of CRN when compared with those of HD-WLE and VCE, although DCE numerically outperformed HD-WLE and VCE. However, procedure time was significantly longer (9.63 minutes) in DCE than in VCE, particularly NBI. Based on previous studies, DCE was significantly superior to SD-WLE. Additionally, a trend favoring DCE over HD-WLE was observed; however, the statistical significance was inconsistent. Several meta-analyses have reported that HD-WLE is not inferior to DCE. VCE, including NBI and iSCAN, failed to improve CRN detection in patients with IBD. However, VCE showed significantly non-inferior performance compared to HD-WLE and DCE, although DCE showed numerically superior performance. VCE have several advantages over DCE. VCE reduced procedure time and cost of dye application. Moreover, it is free of uneven dye staining, which hampers accurate mucosal observation.43 The recently updated SCENIC guidelines recommend DCE for surveillance colonoscopy in patients with IBD if SD-WLE is used. HD-WLE alone is also recommended, considering the disadvantages of DCE application. VCE or DCE is an acceptable method for surveillance colonoscopy in patients with IBD if HD colonoscopy is used; however, it should be performed by well-trained endoscopists. Additionally, targeted biopsy is sufficient if patients do not present with any high-risk features of CRN development, such as a previous history of neoplasia, tubular colon shape, or primary sclerosing cholangitis.44 This recommendation is also supported by European Society of Gastrointestinal Endoscopy (ESGE) guidelines and expert opinion.45,46 Table 4 summarizes the representative studies for CRN detection in IBD using IEE.30-32,35-40 ENDOSCOPIC EVALUATION FOR CHARACTERIZATION OF CRN Chronic inflammation in IBD leads to mucosal distortion and subsequent regenerative changes, which interrupts the adaptation of Kudo pit pattern, as well as prediction of histology and invasiveness of CRN.39 Moreover, the proportion of CRN confirmed by histology among suspicious lesions is approximately 15% during colitis surveillance, which poses difficulty in discriminating neoplastic lesions from nonneoplastic and regenerative changes.38,47 Several studies assessed the endoscopic features and predictors to differentiate CRN using DCE to enhance CRN characterization in IBD. Sugimoto et al.48 demonstrated that flat or superficial elevated lesions, red discoloration, and left-side location were associated with high-grade dysplasia (HGD) using DCE as well as NBI with magnifying colonoscopy, if necessary. Furthermore, pit pattern I or II was not observed in HGD, whereas pit pattern IV and pit pattern IIIL were frequently detected in superficial elevation and flat lesions, respectively. Carballal et al.47 reported that proximal location (OR, 1.86; 95% CI, 1.02–3.40; p=0.04), Kudo pit pattern III–V (OR, 5.05; 95% CI, 2.58–9.88; p=0.001), polypoid morphology (OR, 2.80; 95% CI, 1.57–5.01; p=0.001), and loss of the innominate lines (OR, 1.95; 95% CI, 1.06–3.58; p=0.003) were associated with CRN in IBD. In contrast, a recent retrospective study that used DCE to identify CRN reported that Kudo pit pattern ≥III had a low correlation with CRN with an area under the curve (AUC) of 0.649.49 However, Kudo pit patterns I and II presented a high negative predictive value of 92% even for non-experts, indicating that it can be used to select lesions for target biopsy.49 When VCE was assessed to characterize CRN in patients with IBD, Matsumoto et al.50 showed that the tortuous surface pattern observed by magnifying NBI might be useful for identifying CRN during surveillance colonoscopy for UC. A pilot study by Bisschops et al.51 evaluated the diagnostic accuracy and interobserver agreement of Kudo pit pattern using HD-DCE or non-magnified HD-NBI to assess CRN in UC surveillance. They found that Kudo IIIL, IIIs, IV, or V pit patterns had a sensitivity of 77% for identifying CRN. This study also found that HD-NBI had significantly better interobserver agreement for distinguishing between neoplastic and nonneoplastic pit patterns than HD-DCE (κ=0.653 vs. 0.495, pp51 Moreover, when surveillance colonoscopy was performed using HD-WLE, HD-DCE, and iSCAN, Kudo pit pattern II, III–IV, and V (OR, 21.50; 95% CI, 8.65–60.10) also significantly increased CRN prediction with right side location (OR, 6.52; 95% CI, 1.98–22.5).39 To effectively distinguish neoplastic lesions from nonneoplastic lesions using advanced endoscopic technologies, a new endoscopic classification of visual characteristics, the Frankfurt advanced chromoendoscopic IBD lesions (FACILE), was developed and validated.52 FACILE was generated through uni- and multivariate analysis of various endoscopic features including morphology (polypoid, nonpolypoid), surface architecture (roundish, villous-regular, villous-irregular, and irregular/nonstructural), vessel architecture (nonvisible, regular, and irregular/nonstructural) as well as inflammation within the lesion (yes or no) observed by HD-WLE, DCE, and VCE, including iSCAN and NBI. Irregular vessel architecture (OR, 3.49; 95% CI, 1.74–7.10), nonpolypoid lesion (OR, 3.13; 95% CI, 1.32–7.25), irregular surface patterns (OR, 8.89; 95% CI, 3.21–25.96), and signs of inflammation within the lesions (OR, 2.42; 95% CI, 1.24–4.79) were significant predictors of CRN. Additionally, the sensitivity, specificity, and AUC of this classification for predicting CRN were 94%, 51%, and 0.76, respectively. Recently, Cassinotti et al.53 evaluated the differentiation performance of FICE using Kudo classification; the sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio of the Kudo classification were 91%, 76%, 3.8, and 0.12, respectively. From this study, the authors further studied new endoscopic classification (FICE-KUDO/IBD) specific for FICE, the modified Kudo classification, and included pit patterns and inflammatory markers to differentiate visible colonic lesions. Comparing the performance of FICE-KUDO/IBD with that of SD-WLE, FICE-KUDO/IBD classification was more accurate in identifying neoplastic lesions, with a sensitivity of 93% and specificity of 97%. Furthermore, FICE-KUDO/IBD had a significantly better nonpolypoid lesions detection rate than SD-WLE (8 vs. 7, p=0.016).54 Table 5 summarizes the representative studies characterizing CRN in IBD using IEE.47-51,53,54 CONCLUSIONSPrecise endoscopic assessment of disease activity is fundamental for the optimal treatment of IBD. Furthermore, accurate detection and optical diagnosis of suspicious neoplastic lesions during surveillance colonoscopy are crucial to avoid unnecessary biopsies and reduce surgical treatment due to delayed carcinoma diagnosis in patients with IBD. With recent progress in IEE technologies, VCE platforms such as NBI, iSCAN, FICE, and BLI/CLI systems were developed and adopted in colonoscopic examinations to improve the performance of endoscopic assessments in patients with IBD. Although advanced VCE technologies such as NBI have limitations in evaluating vascular surface patterns in patients with hemorrhage, VCE shows potential for predicting histological remission and clinical outcomes in patients in an endoscopically quiescent state. Additionally, several studies, including meta-analyses, have reported that HD-DCE, HD-VCE, and HD-WLE have comparable performances in detecting CRN during surveillance colonoscopy, although a trend favoring DCE over HD-WLE was observed. Moreover, VCE showed the possibility of differentiating nonneoplastic lesions from neoplastic lesions. To establish IEE techniques, especially VCE, as standard-of-care in managing IBD, to tailor therapeutic strategies, and to detect and manage CRN earlier in patients with IBD, growing evidence of the widespread application of IEE technologies in a large IBD population is warranted.
Table 1.Summary for currently available IEE techniques
IEE technique Mechanism Strength Weakness Dye-chromoendoscopy Absorptive dye: crystal violet, methylene blue Enable to inspect details of cellular surface as the dye is absorbed by epithelial cells. Enhance the detection of mucosal abnormalities such as inflammation or dysplastic changes based on the degree of stain uptake and differentiation of dysplastic changes through precious observation of pit pattern. (1) Additional costs for the equipment needed for dye spraying. (2) Time consuming procedure. (3) Incomplete or uneven mucosal dye coverage. (4) Need for experience to interpret the suspicious lesion. Contrast dye: indigo carmine Emphasize irregularities of mucosal surface by being pooled in pit or innominate groove without absorption. Facilitate to demarcate mucosal abnormalities. Virtual chromoendoscopy NBI Use two narrow-band of blue (415 nm) and green light (540 nm), which are absorbed by hemoglobin in blood vessels to enhance mucosal surface and capillary patterns. (1) Easily be activated at a push of a button. (2) Helpful for inspecting the mucosal vascular pattern. (1) Poor illumination intensity of the first generation NBI system leads to dark and harsh images, especially in GI tract with a wide lumen. (2) Need training to achieve competence for analyzing surface and vascular patterns. (3) Disagreement between observers in diagnosis using NBI. FICE Computed spectral image processing that reconstructs dedicated wavelengths resulting in improved visualization of mucosal structures and microvasculature Improve analysis of pit pattern and mucosal junction between normal and pathologic lesion. (1) Difficulty in providing high-contrast images of microvessels under white light. (2) Difficulty to choose FICE channel according to clinical cases. (3) Require advanced endoscopic technologies and experience. iSCAN Digital post-processing image enhancement technology, which provides digital contrast to endoscopic images using three functions: surface enhancement, contrast enhancement, and tone enhancement. (1) Enhance the visualization of the mucosal structure, vascular patterns, subtle color changes. (2) Facilitate detection and characterization of the lesions. (3) Red remains the predominant blood vessel color, unlike NBI. (1) Need further validation. (2) Require additional cost to install platform, advanced endoscopic technologies, and experience. BLI Enhance blue-violet light and simultaneously reduce white light components using semiconductor laser beam to create high contrast images. (1) Emphasize the contrast between blood vessels and surrounding tissues. (2) Brighter than NBI. (1) Limitations to increase the contrast of vessels in submucosa compared with NBI. (2) Images are slightly darker. LCI Based on BLI technique, expand the color range of reddish and whitish colors to generate brighter images compared to WLE images. (1) Improve the recognition of slight differences in mucosal color compared to conventional WLE by reallocating the acquired color information to a color space. (2) Special training is not required because LCI images are similar to the color enhanced images of WLE images. Need further validation. Table 2.Paddington international virtual chromoendoscopy score
Mucosal architecture 0. No mucosal defect. I. Microerosion or crypt abscess. II. Erosions size <5 mm. III. Ulcerations size >5 mm. (A) Continuous/regular crypts. 1: Discrete. 1: Discrete. 1: Discrete. (B) Crypts not visible (scar). 2: Patchy. 2: Patchy. 2: Patchy. (C) Discontinuous and or dilated/elongated crypts. 3: Diffuse. 3: Diffuse. 3: Diffuse.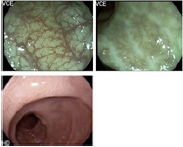
 Vascular architecture
0. Vessels without dilatation.
I. Vessels with dilatation.
II. Intramucosal bleeding.
III. Luminal bleeding.
(A) Roundish following crypt architecture.
(A) Roundish with dilatation.
(A) Roundish with dilatation.
(A) Roundish with dilatation.
(B) Vessels not visible (scar).
(B) Crowded or tortous superficial vessels with dilatation.
(B) Crowded or tortous superficial vessels with dilatation.
(B) Crowded or tortous superficial vessels with dilatation.
(C) Sparse (deep) vessels without dilatation.
Vascular architecture
0. Vessels without dilatation.
I. Vessels with dilatation.
II. Intramucosal bleeding.
III. Luminal bleeding.
(A) Roundish following crypt architecture.
(A) Roundish with dilatation.
(A) Roundish with dilatation.
(A) Roundish with dilatation.
(B) Vessels not visible (scar).
(B) Crowded or tortous superficial vessels with dilatation.
(B) Crowded or tortous superficial vessels with dilatation.
(B) Crowded or tortous superficial vessels with dilatation.
(C) Sparse (deep) vessels without dilatation.
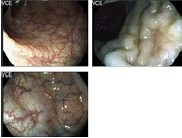
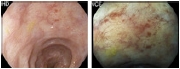
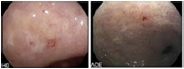
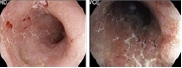 Table 3.
Table 3.
Representative studies evaluating disease activity assessment in IBD using IEE
Study Study design Study population Aim Colonoscope technique Outcomes Kudo et al. (2009)15 Single center prospective study 30 Inactive or mild UC (157 colorectal segments) To assess correlation between MVP and the histologic grade of inflammation Conventional vs. NBI colonoscopy Obscure MVP under NBI colonoscopy was associated with acute inflammatory cell infiltrates (26% vs. 0%, p=0.0001), goblet cell depletion (32% vs. 5%, p=0.0006), and basal plasmacytosis (2% vs. 21%, p=0.006). Sasanuma et al. (2018)16 Single center prospective study 52 UC: left-sided or total-colitis type UC and achieved clinical remission with an endoscopic Mayo score of 0 or 1 To assess the relationships of magnified NBI with histological disease activity and prognosis White light vs. magnified NBI Magnified NBI findings such as blood vessels shaped like vines showed a good relationship with pathological findings (p<0.01). Neumann et al. (2013)17 Prospective randomized controlled study 78 Mild or inactive IBD (36 UC, 42 CD) To assess whether iSCAN has the potential to enhance assessment of disease severity and extent compared to HD-WLE HD-WLE, VCE (iSCAN) iSCAN significantly improved the diagnosis of the severity (53.85% vs. 89.74%) and extent (48.71% vs. 92.31%) of mucosal inflammation in patients with IBD. Average duration of the examination was comparable between HD-WLE and iSCAN (18 min vs. 20.5 min). Iacucci et al. (2015)18 Single center retrospective cohort study 78 UC To create a more refined histological and endoscopic criteria based on this novel technique in order to redefine inflammatory activity and mucosal healing HD-VCE (iSCAN), WLE Of those with Mayo endoscopic subscore of 0, 30.4% had an abnormal mucosal pattern and 73.9% of them had an abnormal vascular pattern on iSCAN. Iacucci et al. (2017)19 Single center prospective cohort study 41 UC and 9 control To investigate the use of iSCAN-OE in the assessment of inflammatory changes in UC VCE (iSCAN-OE) iSCAN-OE accurately identified mucosal inflammation with the accuracy of 68% to 80% and correlated well with ECAP (r=0.70, p<0.001) and RHI (r=0.61, p<0.01). Iacucci et al. (2021)22 Multicenter prospective international study 307 UC
留言 (0)