“Antimicrobial Resistance” (AMR) is a condition in which bacteria, viruses, fungi, and parasites evolve over time and cease to respond to drugs, rendering outbreaks extremely difficult to treat and raising the risk of disease transmission, life-threatening sickness, and death. Each year, more than 700,000 individuals lose their lives due to AMR. The emergence of antibiotic-resistant superbugs, or AMR, has served as a global wake-up call. Before the outbreak of Coronavirus disease-19 (COVID-19), one of the top 10 risks identified by the World Health Organization (WHO) is AMR, which is also known as the “silent pandemic.” The United Kingdom (UK) government has appointed an economist, Lord Jim O’Neill, to conduct a strategic assessment of how to best combat AMR in the UK. According to his report, if no action is taken, then AMR will result in 10 million deaths annually by 2050.[1-3]
The COVID-19 pandemic has highlighted the significance of enhancing hygienic practices as well as infection control, particularly in low- and middle-income nations. Antimicrobials have often been given to patients in the hospital who had COVID-19 symptoms to lessen their risk of developing secondary bacterial infections, which increases the prevalence of resistant strains. Increased usage of disinfectants, such as hand sanitizers and surface cleaners, is projected to result in higher instances of resistant pathogens in the upcoming years.[2,3]
In the agricultural and medical sectors, the indiscriminate utilization of antimicrobial agents (AMAs) is getting worse. Like all other organisms, pathogens evolve by adapting to new environmental factors. When a particular community of microbes is exposed to an antimicrobial, vulnerable pathogens perish while resistant pathogens live. The introduction of new antibiotics in the market was much easier and quicker a century back than it is today. After the initial euphoria, it became apparent that bacteria are capable of developing, acquiring, and disseminating a wide range of resistance mechanisms. As antimicrobial resistance increases internationally, causing diseases that are difficult to treat and ultimately lead to mortality. Antibiotics are becoming less and less effective. For the treatment of carbapenem-resistant Gram-negative infections, new antibiotics are urgently required. AMR reduces productivity by necessitating prolonged hospitalization and thus more expensive, intensive treatment.[3,4]
HISTORY OF ANTIMICROBIAL AGENTS (AMAS)Bacterial infections were the leading cause of death in the developed world up until the turn of the 20th century. Several civilizations utilized various moulds and plant extracts to cure diseases. In 1910, Paul Ehrlich introduced the arsenic-based synthetic drug “Salvarsan” for treating syphilis caused by Treponema pallidum.[5] Later in 1928, Alexander Fleming discovered “Penicillin.”[6] In 1930, Waksman defined antibiotics as “A compound made by a microbe to destroy other microbes” and Actinomycetes, which inhabit soil, were recognized by him as being active producers of antimicrobial substances. Neomycin and streptomycin, the first antibiotics effective against tuberculosis, were among the several antibiotics produced by soil-dwelling Actinomycetes that Waksman identified. In his ground-breaking research, Waksman identified the genus Streptomyces as a prolific natural product generator.[3,7] The “golden age” of antibiotic discovery, which lasted from the 1940s through the 1960s, was started by Waksman’s efforts but the emergence of AMR has lowered their efficacy. The cephalosporins were initially isolated from the fungus Cephalosporium acremonium cultures by the Italian scientist Giuseppe Brotzu in 1945.[8]
Vancomycin was eventually obtained from Streptomyces orientalis, which became available to patients in 1958.[9] Antibiotic resistance was by that period becoming a problem, therefore researchers looked for novel ways to enhance existing drugs to overcome it. The first penicillinase-resistant β-lactam antibiotic, methicillin, was created by Beecham in 1959. ampicillin was introduced around 1961.[10,11]
As a by-product from Streptomyces clavuligerus culture, beta-lactamase inhibitors were discovered in 1976.[12] These resulted in the development of thienamycin, derived from Streptomyces cattleya in 1976, which was the precursor for the carbapenems, and clavulanic acid, which when coupled with amoxicillin, produced co-amoxiclav.[11,12] Around 1987, a new class of antimicrobials had been discovered and introduced to the market. Since then, there has not been much advancement in this area, and there are not many novel antimicrobial classes in the pipeline right now. The paucity of antibiotics for Gram-negative bacteria is particularly concerning. The timeline of the major antibiotics’ discoveries and the mode of action is depicted in [Figures 1 and 2].

Figure 1:: Timeline of antimicrobials discovery.[3]
Export to PPT

Figure 2:: Antimicrobials classification on the basis of mode action.[3]
Export to PPT
MICROORGANISMS INVOLVED IN AMRMicrobes through genetic mutations acquire resistance to combat with antibiotics and thus maintain their survival. When in 1941 “Penicillin” was first commercially available in the market then Streptococci, Staphylococci, and Gonococci were apparently found to be resistant first; then in 1942, Staphylococcus aureus was found to be resistant to penicillin and later in 1960, it was found resistant to methicillin.[3] Later on, other microbes gradually developed resistance to various antibiotics including carbapenem which is considered as a last resort to treat gram-negative infections.[13] The WHO in 2017 published a list of high-priority pathogens that were divided into high, medium, and critical priority[14,15] [Table 1].
Table 1:: Priority list for bacteria released by the World Health Organization in 2017.[15,17] Critical High Medium Acinetobacter baumannii Enterococcus faecium Streptococcus pneumoniae Pseudomonas aeruginosa Staphylococcus aureus Haemophilus influenzae Enterobacteriaceae Helicobacter pylori Shigella spp. Campylobacter spp. Salmonellae Neisseria gonorrhoeaeThe WHO is more concerned about “ESKAPE” (“Enterococcus faecium, S. aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.”) pathogens as they have emerged as multidrug resistance (MDR), pan drug resistance and extensively drug-resistant, infections caused by them are very difficult to treat.[16]
The diseases caused by fungi have been neglected for so long that we barely comprehend the extent of the problem. The rising problem of invasive fungal diseases prompted the establishment of the “Fungal Priority Pathogens List” by the WHO on October 25, 2022, which includes 19 fungi that pose the highest threat to public health. Patients who are critically unwell and those with serious underlying immune system-related disorders usually have these fungus infections in their invasive forms[17] [Table 2].
Table 2:: “Fungal Priority Pathogens List” by the World Health Organization.[17] Critical High Medium Cryptococcus neoformans Candida glabrata Scedosporium spp. Candida auris Histoplasma spp. Lomentospora prolificans Aspergillus fumigatus Eumycetoma causative agents Coccidioides spp. Candida albicans Mucorales Candida krusei Fusarium spp. Cryptococcus gattii Candida tropicalis Talaromyces marneffei Candida parapsilosis Pneumocystis jirovecii Paracoccidiodes spp.Human immunodeficiency virus (HIV) genetic variations limit the capacity of medications to stop the virus from replicating, which is the cause of HIV treatment resistance. Due to the advent of drug-resistant viruses, all antiretroviral drugs, even those belonging to more recent pharmacological classes, run the possibility of becoming partially or completely inactive. The emergence and dissemination of drug resistance, notably to artemisinin and partners on Artemisinin combination therapies, the first-line treatment advised by the WHO, pose a danger to the control and eradication of malaria. Three of the five malaria species that afflict humans, Plasmodium vivax, Plasmodium falciparum, and Plasmodium malariae have been discovered to be resistance to antimalarial drugs.[18]
RESISTANT MECHANISMAMR has evolved to be a major cause of morbidity and mortality throughout the world. It is anticipated that overall awareness of such strategies eventually results in more effective treatments for infectious diseases.[19] There are various strategies such as intrinsic resistance, horizontal gene transfer, biofilm formation and mutations etc. through which microbes have adopted resistance against antimicrobial medicines [Figure 3].

Figure 3:: Resistance mechanism of bacteria.
Export to PPT
Following are the methods through which microbes acquired resistance to antimicrobial drugs:
Porin mediatedThe plasma-membrane of Gram-positive bacteria is encased in a hard, stiff mesh known as the “cell wall.” Gram-negative bacteria have a thin cell wall and an outer membrane, which is a second lipid membrane. The outer membrane comprises proteinaceous porin channels that permit the entry of many substances, including antibiotics.[20] Choi and Lee (2019) in their research on Escherichia coli’s outer membrane porins found that OmpF mutant was resistant to several antibiotics including β-lactams.[21] Liu et al. (2022) found that the deletion of the hopE and hopD porin genes lowered the amount of streptomycin in the Helicobacter pylori and, hence, restored the expression of the resistant gene, which was suppressed by streptomycin in the wild-type strain.[22] A study of the outer membrane proteins of E. coli and K. pneumoniae isolates done by Khalifa et al. (2021), revealed that 93.3% and 95.7%, respectively, had porins that had been deleted or altered. In addition, frameshift mutations in some isolates’ porin genes were also found by sequence analysis.[23]
Overexpression of efflux pumpThe transporter protein referred to as “efflux pumps” is found in bacterial cells and is located in the cytoplasmic membrane. These are required to evacuate various drugs, dyes, and detergents. Mutations in the efflux pump system cause these pumps to be overexpressed, which minimizes the accumulation of antibiotics in the bacterial cell and, thus, helps bacteria get rid of these antibiotics.[24] According to Pandey et al. (2020), fluconazole resistance in Candida tropicalis, which is responsible for approximately 40% of candidemia, is acquired by the overexpression of efflux pump transporter genes and ERG11 mutations.[25]
In the K. pneumoniae strain (KPN142), which is pan-drug resistant, the overexpression of phoPQ causes the resistance to colistin B, and the overexpression of the AcrAB-TolC efflux pump facilitates the resistance to the majority of conventional medical antimicrobials, according to Lv et al., 2021.[26]
Bankan et al. (2021) in their research study found that 19 out of the 42 A. baumannii isolates that were tigecycline-resistant exhibited efflux pump activity. The adeB gene was expressed by all 19 strains.[27]
Plasmids with antibiotic resistance genes“Plasmids” are extra-chromosomal double-stranded deoxyribonucleic acid (DNA) within a bacterial cell and replicate independently. Plasmids contain various antibiotic-resistance genes that are beneficial to bacteria for their survival. These plasmids are capable of transfer resistance genes through horizontal gene transfer and during conjugation. Cross-species transfer within different groups of bacteria is also possible due to plasmids. Plasmids are ideal vectors for the spread of AMR as they are capable of acquiring novel genes through mobile genetic elements such as transposons or insertion sequences.[28]
Oxacillinase-encoding genes (OXA-23, OXA-24, and OXA-58) and their variants that confer carbapenem drug resistance are acquired by A. baumannii through plasmids. ColE1-type replication is used by K. pneumoniae plasmids that are 25 kb or less in size. Many of these plasmids contained the transposon Tn1331 and its variants.[29] Given the scarcity of available antibiotics to treatment, hospital-associated infections are frequently plagued by resistance to third-generation cephalosporins, which is frequently caused by extended-spectrum beta-lactamases (ESBLs). The ESBL genes are frequently found on large plasmids that move horizontally between Enterobacteriaceae isolates and species. Hawkey et al., 2022 in their research study, sequenced the genome of K. pneumoniae isolates and identified 25 distinct plasmids in which plasmid A was found to be carried blaCTX-M-15 resistant gene. It caused 50% of all ESBL occurrences during the 1st year of their study and was spread at least 4 times into various Klebsiella spp.[30]
MutationWhen microbes are exposed to higher concentrations of antimicrobials, then natural selection plays a role and mutations occur in the genome that gives rise to resistant pathogens, while wild-type strains are eliminated from the environment. These resistant variants can transfer mutant genes to other microbes naturally during conjugation or through spontaneous mutation and may provide intrinsic resistance. For example, A. baumannii has the OXA-51 intrinsic resistant gene that is only specific for it.[31]
In their research on Elezabethkingia anophelis, Lin et al. (2018) observed that out of 67 isolates, 11 isolates showed mutations in DNA gyrase subunit A that indicated high levels of fluoroquinolone resistance.[32] Bachmann et al. (2020), through Sanger sequencing, found mutations at the 23S ribosomal ribonucleic acid (rRNA) locus (macrolide resistance-associated mutations), as well as in the parC and gyrA genes (quinolone-associated mutations) of Mycoplasma genitalium that causes urethritis.[33] According to Nagy et al., 2022 the gmhD genetic variant of Shigella sonnei (strain 4351) caused an enhanced resistance to the cephalosporin and macrolide. This is due to frameshift mutation in the genome of S. sonnei.[34]
Antibiotic-modifying enzyme or Antibiotic degrading enzymeA vast number of enzymes inactivate antimicrobials by modifying or degrading their structure. All penicillin-binding proteins (PBPs) are targeted by β-lactam antibiotics at their C-terminus [Figure 4].

Figure 4:: Antibiotic resistance due to mutations in penicillin-binding protein.
Export to PPT
Gram-positive bacteria develop resistance to β-lactam antibiotics as a result of mutations in PBPs. PBP2a is a mutant form that is encoded by the MecA gene and exhibits only transpeptidase activity, thus conferring resistance to S. aureus against methicillin.[35] The methylation of the 16S rRNA A-site by bacterial 16S rRNA methyltransferases, which impairs the potential of antimicrobials to bind to the ribosome, provides resistance to aminoglycosides.[36]
Antibiotic modifying enzymes can be divided in three parts –
Hydrolases – Esterases, β-lactamases, Epoxide hydrolases
Transferases – Acetyltransferases, Phosphotransferases, Glycosyltransferases, Neucleotidyltransferases, etc.
Redox enzymes – Monooxygenases, Lyases [Figure 5].
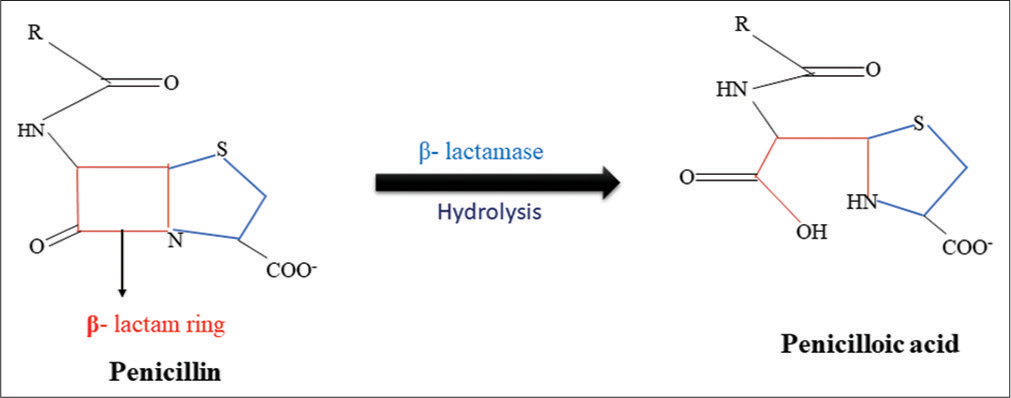
Figure 5:: Hydrolysis of β-lactam ring by β-lactamase.
Export to PPT
β-lactam antibiotics have amide bond in the β-lactam ring which is hydrolyzed by β-lactamases. Esterases are responsible for the degradation of the lactone ring that leads to resistance against 14- and 15-membered macrolides. Bacteria are mainly resistant to chloramphenicol due to the production of chloramphenicol acetyltransferases. These enzymes catalyze the addition of the acetyl group of acetyl-CoA to the 3-hydroxyl group of chloramphenicol or its synthetic counterparts (Azidamphenicol and Thiamphenicol), which prevents adhering to ribosomes. The 2’-OH group of the macrolide ring is glycosylated by macrolide glycosyltransferases that causes inactivation of macrolides. By modifying the hydroxyl group, several enzymes deactivate rifamycin. Several tetracyclines, and tigecycline, are resistive to monooxygenase.[37]
Biofilm formationBacteria shield themselves from antibiotics, disinfectants, and host inflammatory responses by establishing a biofilm. Biofilm is a complex microbial structure that attaches to a surface and includes a wide range of microbes. Especially in nosocomial conditions, it is among the major causes of infection recurrence [Figure 6].[38]

Figure 6:: Biofilm formation by microbes.
Export to PPT
Antimicrobial compounds are significantly less effective against microorganisms in biofilms than they are against planktonic forms.[39] Both Gram-positive bacteria such as S. aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, and Gram-negative bacteria such as P. aeruginosa, K. pneumoniae, A. baumannii, and E. coli, are capable to form biofilm.[40]
Quorum sensing (QS) mechanisms are used by microbes to interact with one another within biofilms. These systems rely on chemical signals. Communication affects density-based pathophysiology, cellular processes, genetic material transfers between cells, dietary uptake, motility, and the production of secondary metabolites. Oligopeptides are used by the Gram-positive bacteria as a signaling molecule whereas Gram-negative bacteria utilize homoserine lactone dependent QS system.[41]
Dimitrova et al., 2021 in their research work on swine feces and lagoons found 16 E. coli strains, of which 31.2% exhibited strong biofilm formation, 37.5% formed moderate biofilm whereas 25% formed weak biofilm and 87.5% were MDR.[42] Biofilm of K. pneumoniae can promote colonization in the gastrointestinal, urinary, and respiratory tracts as well as the emergence of nosocomial infections such as ventilator associated pneumonia in immunocompromised patients.[43] It is challenging to anticipate which compounds may develop into novel therapeutic antifungal drugs as Candida albicans biofilms are still not very susceptible to therapeutic agents already in use.[44]
OTHER CAUSES OF AMR Human wasteThe resistant strains develop when unmetabolized or partially digested pharmaceuticals are discharged into health-care waste. Chaudhary and Uddin, 2022, found cefixime-resistant bacteria in the effluents of Chittagong Medical College Hospital, Bangladesh.[45] Antibiotics, antifungals, and pathogens can be found in feces. The sewage treatment plants aren’t really designed primarily to get rid of them, and due to overflow, leakage, or untreated wastewater, contamination ultimately results in various diseases and the proliferation of resistant strains might happen.[46,47]
Agriculture industryIn the agriculture industry, antibiotics are used to prevent crop diseases and enhance the yield of crops. According to Miller et al., 2022 phytopathogens are controlled by the application of fungicides, antibacterial drugs, and other pesticides. These pathogens have become more resistant to oxytetracycline, copper-based compounds, streptomycin, and some fungicides as a consequence of their application. In Europe, a recent increase has been seen in the prevalence of Aspergillus fumigatus, which causes human aspergillosis and is resistant to triazole.[48,49]
One of the most frequently used chemicals in agriculture is pesticide. According to Liao et al., 2021 three commonly used herbicides, that is, glufosinate, glyphosate, and dicamba enhanced the incidence of mobile genetic elements and AMR genes in soil microbiomes. This might exacerbate the issue of worldwide antibiotic resistance in agricultural ecosystems.[50]
Aquaculture industryAquaculture aids in food security and improves the state of the world economy. Untreated or inadequately managed waste and effluents from many sources are disposed of carelessly, allowing numerous pollutants, such as bioactive substances and unmetabolized antibiotics, to enter the environment.[51]
Antimicrobials such as aminoglycosides, macrolides, quinolones, tetracycline, and sulphonamides are used in aquaculture systems for both treatment and prevention. As a consequence, the microbes in the vicinity developed AMR, and other microbes can become resistant through horizontal gene transfer, allowing these resistant microbes to enter humans through the food chain and cause disease. Approximately 80% of the medicines ingested are not absorbed and end up in the surroundings through feces. After being absorbed, antimicrobials are then eliminated by the urine and other secretions and accumulate in the sediments, increasing their concentration in the aquarium, which causes resistance as microbes are now under selection pressure for survival.[52]
Animal husbandryIn animal husbandry, AMAs are used to treat various diseases as well as act as growth promoters.[53] Developing countries adopted intensive farming due to the high demand for animal proteins. Resistant pathogens that are associated with animals are a great threat to humans as they can be easily transmitted from animals to humans through the food chain and are widely distributed in the environment. Inadequate government policies, as well as the limited adoption of infection control strategies, all contribute to the continued use of non-essential antimicrobials in animal husbandry.[54]
Salmonella are zoonotic microorganisms that exhibit antibiotic resistance, particularly to drugs recovered from farm animals. Salmonella can be found in the native gut flora of chickens, hence makes them a reservoir of these bacteria. Animal feed is manufactured in underdeveloped nations in an unregulated way without veterinary oversight or microbiological quality testing. Furthermore, some feed producers include antibiotics in poultry feeds to ward off diseases and flock attrition. These procedures might promote antibiotic-resistant pathogens in the intestinal flora of chickens.[55] Several antibiotics used in veterinary medicine possess the same mode of action as those used to combat bacterial infections in humans, and if not fully metabolized, animal manure will become a source of antibiotic contamination and reservoir for MDR pathogens in the soil.[56] According to Wu et al., 2022, sewage sludge, chicken, swine, and cattle manure are the major sources of contamination for sulphonamides, tetracyclines, fluoroquinolones, and associated ARGs in agricultural soils.[57]
Overuse and misuse of antibioticsAccording to Jani et al., 2021, genomic variability as well as the indiscriminate use of pharmaceuticals enable microbes to adjust to the effects of antimicrobials, resulting in a sharp rise in AMR. The reasons behind the increase in treatment failure and the expansion of AMR include self-medication, the availability of antibiotics over the counter, and the prescription of broad-spectrum antibiotics.[58]
Several people frequently use medicines without a prescription, and if they are successful in overcoming a specific ailment, they may employ the same medications to treat other diseases in the future. They may also advise others to do the same. Many folks skip follow-up appointments and do not finish their prescriptions as their doctors have advised.
STRATEGIES TO OVERCOME AMRThe CDC has granted $22 million to 28 organizations across the globe to start two new networks that will combat health-care-associated infections and antimicrobial resistance.[59] The implementation of strategies that avoid any possible exposure of pathogens to antibiotics in non-clinical environments involves cooperation between clinicians, researchers, and policymakers. To combat pathogenic microbes, it is indeed clinically important to use novel and quasi therapies, which are becoming more prevalent in pathogenic bacteria due to increased AMR[60] [Table 3].
Table 3:: Strategies to overcome antimicrobial resistance.
S. No. Strategies against resistance mechanism References 1. Combination drug therapy Bianco et al., 2022; Henson et al., 2016; Li et al., 2019 2. CRISPR technology Rodrigues et al., 2019; Dong et al., 2018 3. Nano-drugs delivery Kang et al., 2017; Bruna et al., 2021 4. Bacteriophage therapy Yazdi et al., 2019; Yang et al., 2020; Roach et al., 2017 5. Vaccines Kaufhold et al., 2019; Barchitta et al., 2022 6. Antimicrobials peptides Liu et al., 2018 7. Faecal microbiota transplantation Freedman et al., 2014; Ueckermann et al., 2020; Bilinski et al., 2017 8. Use of Probiotics and Prebiotics Buyukeren et al., 2020 9. Antimicrobial stewardship practices World Health Organization, 2015 10. Global Surveillance approaches World Health Organization, 2015 11. Awareness and Education Marvasi et al., 2021 Combination drug therapyCombination therapy has been developed by specialists for use against pathogens especially MDR microbes. Combination therapy requires the integration of two or more pharmaceuticals to boost or enhance each one’s efficacy.[61] Carbapenem drugs are considered a last resort to treat Gram-negative infections, but global resistance to these drugs is a worrisome situation as pathogens produce lactamase enzyme as a defense mechanism. Bianco et al., 2022, reported that when avibactam, vaborbactam, and relebactam were combined separately with cefiderocol, the minimal inhibitory concentration (MICs) of cefiderocol showed a 4–256 reduction against carbapenem resistant K. pneumoniae, which was harboring virulent Klebsiella pneumoniae carbapenemase (KPC)-resistant genes. They demonstrated that cefiderocol MICs of K. pneumoniae strains that were harboring blaKPC-41, blaKPC-31, blaKPC-53, and blaKPC-66 genes dropped by 4–64-fold in response to tazobactam.[62]
Another study has been done by Henson et al. (2016) to investigate synergistic effect of daptomycin in combination with piperacillin-tazobactam and ampicillin-sulbactam against methicillin resistant S. aureus and found that six out of eight Methicillin-resistant Staphylococci strains showed synergistic effect.[63] Li et al. (2019) in their study mentioned that when D-penicillamine is combined with fluconazole then it showed synergistic effect against C. albicans as well as against its biofilms that formed within 12 h in vitro.[64]
CRISPR technologyIt is becoming more challenging for us to tackle infectious diseases and generate new pharmaceuticals as a result of the emergence of antibiotic-resistant microbes. Innovative drugs that can kill MDR pathogens and distinguish between beneficial and dangerous species may be developed utilizing CRISPR-Cas technology.[65] CRISPR-Cas9 is also known as “RNA-guided-DNA cutter.” The Cas system encodes minute phage genome sequences into the bacterial genome to launch a defense after bacteriophage invasion. Given that Cas9 has nuclease activity, it is indeed possible to program it to produce a highly specific sequence.[66]
Target specificity, which enables differentiation between commensal and harmful microorganisms, is an important feature of CRISPR/Cas. Guide RNAs can be designed to specifically target pathogen-specific genes, antibiotic resistance genes, and virulence genes. This ensures that innocuous strains are maintained while pathogenic variants are terminated in a species-specific approach. According to research conducted by Rodrigues et al., 2019, the presence of antibiotic-resistant E. faecalis is reduced significantly by the administration of CRISPR-Cas antimicrobial drugs inside the gut of mice. They revealed that E. faecalis donor strains carrying CRISPR-Cas antimicrobials are resistant to in vivo acquisition of resistance genes.[67]
In their research study, Dong et al. (2018), created a host-independent conjugative plasmid and employed an engineered CRISPR/Cas9 system to eliminate bacteria carrying the mCR-1 plasmid. They discovered that conjugative plasmids can cause the recipient cell to develop resistance against the mCR-1-interacting plasmid in addition to serving as a novel tool for eliminating resistant plasmids and making the target bacterium more susceptible to antibiotics.[68]
Nanodrugs deliveryIn order to develop effective therapeutic approaches for MDR planktonic pathogens that form biofilms, nanotechnology offers a new set of tools. Nanoparticles are able to circumvent current resistance mechanisms and might be less likely to promote resistance than traditional antibiotics.[69] Their exceptionally small size and high surface-to-volume ratio enable their unique efficacy in therapeutics. In the treatment of diseases spurred on by intracellular pathogens and MDR isolates, this provides a significant competitive advantage over traditional medications.[70] By effectively delivering the Cr-Nanocomplex to Methicillin-resistant S. aureus, Kang et al., 2017, showed that it can be even more effective at editing the bacterial genome than native Cas9 complexes or traditional lipid-based formulations.[71] According to Bruna et al., 2021, silver nanoparticles (AgNPs) have been promoted as a superior antibacterial agent capable of battling microbes that cause disease both in vitro and in vivo. AgNPs have several, concurrent modes of action, and they have a synergistic effect when combined with antimicrobials to combat pathogens like E. coli and S. aureus.[72]
Bacteriophage therapyUtilizing viruses, phage therapy (PT) tackles bacterial infection. Phages or bacteriophages are the terms for bacterial viruses. PT primarily uses obligately lytic phage to eliminate the associated microbial host while sparing human cells and minimizing the major influence on commensal bacteria that frequently arises from antibiotic use.[73]
“Staphylococcus saprophyticus” is a Gram-positive, nonhemolytic, and catalase-positive bacteria that causes urinary tract infections, especially in females. Yazdi et al., 2019, isolated lytic phage (vB_SsapS-104) against S. saprophyticus and found that it was able to lyse eight out of the nine clinical isolates in vitro received from a hospital in Gorgan, Iran.[74]
Yang et al., 2020 isolated virulent Enterococcus faecalis bacteriophage PHB08. They demonstrated that when used against E. faecalis biofilms, PHB08 and its endolysin lys08 both exhibited antibiofilm activity.[75] According to Roach et al., 2017, the host immune system and bacteriophage should work synergistically for PT to really be effective against an acute respiratory pathogen.[76]
VaccinesVaccines are administered as a preventative measure and are effective before bacteria commence to proliferate after the onset of infection. This significantly lowers the risk that mutation conferring resistance will arise and propagate.[77]
According to Kaufhold et al., 2019, typhoid conjugate vaccines (TCVs) have potential to treat diseases caused by antimicrobial resistant Salmonella typhi.[78] The WHO-recommended TCV was incorporated to Pakistan’s routine immunization schedule on November 15, 2019, making it the first nation in the world to do so.[79] Barchitta et al., 2022, demonstrated that Influenza vaccination coverage in patients over 64 and AMR in strains of E. coli and K. pneumoniae are significantly inversely correlated.[80] Vaccines stimulate the immune system to locate pathogens and mount an immediate and robust immunological counterattack. A phenomenon known as herd immunity allows numerous vaccines to safeguard the population’s unvaccinated individuals who are unable to receive vaccinations. Some effective vaccines also inhibit pathogen colonization in patients, which leads to highly effective herd immunity.[81]
Antimicrobial peptides (AMPs)Tiny peptide known as AMPs is abundantly found in nature. AMPs can suppress a wide variety of bacteria, fungi, parasites, and viruses. They have a promising future in the fields of medicine, food, agriculture, animal husbandry, and aquaculture.[82]
In eukaryotes, AMPs play a significant role in the innate immune system. They are formed by host cells as part of long-term immune surveillance against pathogen invasion. Numerous AMPs have been identified in a wide range of taxa, including microorganisms, amphibians, fish, reptiles, mammals, birds, and invertebrates since the very first AMP was discovered in the chrysalis of the American silkworm. Nisin, which is synthesized by the lactic Streptococci spp. was the first AMP identified in bacteria, and is poisonous to other bacterial species. Copsin, which is derived from the mushroom Coprinopsis cinerea, has bactericidal properties toward certain G
留言 (0)