Int J Biol Sci 2021; 17(15):4493-4513. doi:10.7150/ijbs.66181
Review
Jingjing Huang1,2#, Jin Wang3#, Hua He1,2#, Zichen Huang3, Sufang Wu1,2, Chao Chen3, Wenbing Liu4, Li Xie4, Yongguang Tao5, Li Cong1,2 ![]() , Yiqun Jiang1,2
, Yiqun Jiang1,2 ![]()
1. The Key Laboratory of Model Animal and Stem Cell Biology in Hunan Province, Hunan Normal University, Changsha, 410013 Hunan, China.
2. School of Medicine, Hunan Normal University, Changsha, 410013 Hunan, China.
3. School of Medicine & Holistic Integrative Medicine, Nanjing University of Chinese Medicine, Nanjing, 210013 Jiangsu, China.
4. Department of Head and Neck Surgery, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, 410013 Hunan, P.R. China.
5. Key Laboratory of Carcinogenesis and Cancer Invasion, Ministry of Education, Department of Pathology, Xiangya Hospital, School of Basic Medicine, Central South University, Changsha, 410078 Hunan, China.
#These authors contributed equally to this work.
This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See http://ivyspring.com/terms for full terms and conditions.
Citation: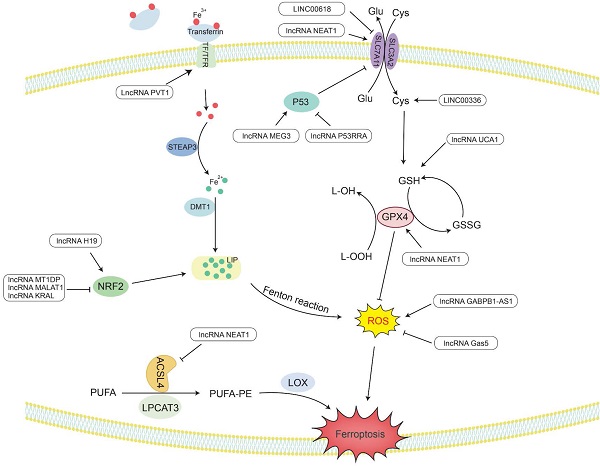
Abnormal lipid metabolism including synthesis, uptake, modification, degradation and transport has been considered a hallmark of malignant tumors and contributes to the supply of substances and energy for rapid cell growth. Meanwhile, abnormal lipid metabolism is also associated with lipid peroxidation, which plays an important role in a newly discovered type of regulated cell death termed ferroptosis. Long noncoding RNAs (lncRNAs) have been proven to be associated with the occurrence and progression of cancer. Growing evidence indicates that lncRNAs are key regulators of abnormal lipid metabolism and ferroptosis in cancer. In this review, we mainly summarized the mechanism by which lncRNAs regulate aberrant lipid metabolism in cancer, illustrated that lipid metabolism can also influence the expression of lncRNAs, and discussed the mechanism by which lncRNAs affect ferroptosis. A comprehensive understanding of the interactions between lncRNAs, lipid metabolism and ferroptosis could help us to develop novel strategies for precise cancer treatment in the future.
Keywords: LncRNAs, Lipid metabolism, Ferroptosis, Cancer
During carcinogenesis, cancer cells usually exhibit a series of metabolic abnormalities that support their need for growth and metastasis. In recent years, increasing evidence has shown that lipid metabolism can be reprogrammed in cancer cells [1, 2], which greatly affects the proliferation, invasion and migration of cancer cells. Lipids are hydrophobic molecules that contain thousands of different types of molecules, including cholesterol, fatty acids (FAs), triacylglycerol (TG) and phospholipids (PL) [3, 4]. These compounds play crucial roles in various biological processes, such as the biosynthesis of membrane lipids, energy metabolism, storage and signal transduction [4, 5]. Cancer cells cause dysregulation of lipid metabolism by affecting the synthesis, uptake, modification, degradation, and transport of these lipids in cells, thus affecting their normal physiological function [3, 6]. In addition, a growing body of studies has found that aberrant lipid metabolism is closely related to ferroptosis, a new type of cell death.
Ferroptosis is a novel form of programmed cell death caused by lipid peroxidation (LPO) [7], which is characterized by the accumulation of LPO products induced by lethal reactive oxygen species (ROS) [8]. Polyunsaturated free fatty acids (PUFAs) are rich in cell membranes and organelle membranes and tend to react with ROS and cause cell damage [9]. Since saturated membrane lipids are not sensitive to peroxidation, highly saturated membrane lipids help protect tumor cells from ROS damage. In tumors, tumor cells can express high levels of antioxidant proteins to reduce ROS levels and prevent the destruction of redox homeostasis [10]. Therefore, the discovery of ferroptosis may provide more ways to treat cancer.
Long noncoding RNAs (lncRNAs) are broadly defined as transcripts of more than 200 nucleotides that are not translated into proteins; lncRNAs were historically considered junk DNA and not taken seriously. However, in recent years, it has been increasingly found that lncRNAs have a place in the occurrence and development of cancer [11]. LncRNAs have complex secondary and tertiary structures and diverse subcellular localizations. On the basis of their subcellular localization, lncRNAs can be classified as nuclear, cytoplasmic, and mitochondrial lncRNAs [12]. Functionally, nuclear lncRNAs seem to preferentially play roles in chromatin remodeling, transcriptional regulation and RNA processing, while cytoplasmic lncRNAs can modulate the stability or translation of mRNA and influence the cell signal cascade [13]. Mitochondrial lncRNAs may act as retrograde signaling molecules, coordinating gene expression in the nucleus and mitochondria and ultimately triggering cellular signaling pathways [14]. Many lncRNAs have been regarded as crucial factors in cancer development by affecting a series of cellular processes, including cell proliferation [15, 16], differentiation [17, 18], metastasis [19, 20] and apoptosis [21, 22], at different levels. Therefore, as a regulatory factor, lncRNAs can regulate tumor lipid metabolism and ferroptosis in carcinoma by mediating the expression of enzymes related to lipid metabolism and ferroptosis-related genes.
Therefore, this article will discuss three major points: 1) several abnormal lipid metabolisms (cholesterol, FAs, TG and PL) regulated by lncRNAs; 2) the effect of lipid metabolism on the expression of lncRNAs; and 3) lncRNAs affecting ferroptosis through lipid metabolism. Elaborating the regulatory relationships of these three factors provides new therapeutic targets and ideas for cancer treatment, which may help alleviate the current clinical difficulties in the treatment of cancer.
Cholesterol metabolism is vital for cellular and systemic biological functions. For instance, cholesterol regulates membrane fluidity and permeability, is an essential component in mammalian cell membranes, and is the precursor of bile acid, cholecalciferol and steroid hormones [23]. Dysregulated cholesterol balance not only promotes cardiovascular disease but also increases the risk of occurrence of other diseases, such as neurodegenerative diseases and cancers [24]. Normally, cellular cholesterol metabolism reflects the dynamic balance between synthesis, uptake, storage and efflux [3]. Over the past decade, the cancer research community has witnessed growing interest in cholesterol reprogramming, including the study of how cancer cells reprogram their cholesterol metabolism, how these reprogramming-derived metabolites consequently promote the progression of cancer and the identities of the key regulators in cholesterol reprogramming [25, 26]. LncRNAs can interact with RNA, chromatin and protein, thus modulating mRNA stability, chromatin structure and the function of proteins (including transcription factors), which makes lncRNAs an important group of crucial factors governing cholesterol metabolism processes in cancer [27] (Figure 1 and Table 1).
LncRNAs regulate cholesterol synthesize and uptakeCholesterol is an indispensable substance in the human body and can be synthesized de novo or ingested from an external source [28]. Acetyl-CoA and NADPH are the basic raw materials for cholesterol synthesis. Acetyl-CoA can be reduced to mevalonate (MVA) under a series of enzymatic reactions with hydroxymethylglutaryl-coenzyme A reductase (HMGCR) as the key enzyme. Then, MVA undergoes decarboxylation and phosphorylation to generate isoprenoids [29]. After the condensation of isoprenoids, 30-carbon squalene is formed. Then, squalene is cyclized to lanosterol under the action of squalene epoxidase (SQLE) [30]. Finally, lanosterol undergoes oxidation, decarboxylation and reduction reactions to generate cholesterol [31]. When the synthesis of cholesterol is insufficient, the low-density lipoprotein receptor (LDLR) can mediate exogenous absorption to maintain the balance of cholesterol in the body [32]. In addition to the increase in cholesterol, which can provide growth requirements for tumor cells, the isoprenoid produced in the MVA pathway can also contribute to the occurrence and development of cancer [33]. Isoprenoids can posttranslationally prenylate small GTP-binding proteins (GTPases), such as the Ras and Rho families, and give them carcinogenic capacity through specific binding to the cell membrane [34]. It is worth mentioning that isopentenyl diphosphate (IPP), the isoprenoid precursor produced in the MCV pathway, can mediate the synthesis of glutathione peroxidase 4 (GPX4) and participate in cholesterol oxidation [35-37]. The hydrogen atom at position C-7 in cholesterol can be taken up by free radicals and then react with diatomic oxygen to produce cholesterol peroxide, leading to lipid peroxidation. However, GPX4 can reduce the occurrence of lipid peroxidation by converting lipid peroxides into lipid alcohols under its antioxidant effect [38, 39]. Therefore, studying the mechanism of the effect of lncRNA on the synthesis and uptake of cholesterol can find ways to inhibit cholesterol reprogramming so that it cannot meet the growth conditions of tumor cells.
It has been reported that lncRNAs can control the expression of HMGCR and LDLR by regulating sterol regulatory element-binding protein 2 (SREBP2) [40]. SREBPs are a class of transcription factors controlling lipid homeostasis by modulating the expression of enzymes required for lipogenesis. SREBP includes three subtypes: SREBP-1a, SREBP-1c, and SREBP-2. It has been indicated that SREBP2 seems preferential to activate the transcription of genes involved in cholesterol synthesis [41-43]. When cholesterol is insufficient, endoplasmic reticulum (ER) cholesterol, as a sensor of intracellular cholesterol homeostasis, can trigger the transport of SREBP2 from the ER to the Golgi apparatus and then to the nucleus to directly regulate the expression of cholesterol-related HMGCR and LDLR [23, 40]. Therefore, directly inhibiting SREBP2 through cholesterol starvation may be an effective strategy against cancer [44]. Yu et al. found that lncRNA SNHG16 is highly expressed in pancreatic cancer and can directly sponge miR-195 to regulate the expression of SREBP2 to accelerate the progression of pancreatic cancer. Moreover, the reduction in pancreatic cancer cell adipogenesis after inhibiting the expression of SREBP2 further proves that lncRNA SNHG16 may fuel the growth of pancreatic cancer cells by regulating the miR-195/SREBP2 axis to provide an energy supply [45].
SQLE is another key controlling enzyme in the MVA pathway. It has been confirmed that SQLE is a metabolic oncogene in breast cancer and is associated with a poor prognosis of breast cancer (BC) [46]. Recent studies have discovered that lnc030 is highly expressed in breast cancer stem cells (BCSCs) and can work with poly(rC)-binding protein 2 (PCBP2) to stabilize the expression of SQLE. PCBP2 is an RNA-binding protein that acts as an intermediary between lnc030 and SQLE. PCBP2 promotes the stability of SQLE mRNA and the production of cholesterol by combining with the lnc030 fragment 301-412 nt and the 3'-UTR of SQLE to form a ternary complex. It has been reported that increased cholesterol synthesis activates PI3K/AKT, the consistent cancer signaling pathway, which is involved in BCSC stemness maintenance [47]. Therefore, lnc030 can promote the occurrence and growth of BC through the SQLE/cholesterol/PI3K/AKT signaling axis.
Figure 1The known functions of lncRNAs in tumor lipid metabolism. The main lipids in tumor lipid metabolism include cholesterol, FAs, TG and PL. LncRNAs regulate the synthesis and catabolism of these four types of lipids through a variety of mechanisms, thereby participating in the occurrence and development of cancer.
 Table 1
Table 1
Summary of lipid metabolism-associated lncRNAs in cancer
LncRNAAssociated type of effectTargetInfluence to targetCancer typeReferenceLncRNA SNHG16Cholesterol synthesizesSREBP2UpPancreatic cancer[45]Lnc030Cholesterol synthesizesSQLEUpBreast cancer[47]LncRNA CASC19Cholesterol uptakeLDLRUpNon-small cell lung cancer[48]LncRNA TINCRDe novo synthesis of fatty acidsACLYUpNasopharyngeal carcinoma[77]LncRNA FLJ22763De novo synthesis of fatty acidsACLYDownGastric cancer[78]LncRNA DNAJC3-AS1De novo synthesis of fatty acidsACC/FASNUpColorectal cancer[87]LncRNA CTD-2245E15. 3De novo synthesis of fatty acidsACC1UpNon-small cell lung cancer[85]LncRNA HAGLRDe novo synthesis of fatty acidsFASNUpNon-small cell lung cancer[94]LncRNA HOTAIRDe novo synthesis of fatty acidsFASNUpNasopharyngeal carcinoma[96]LncRNA PVT1De novo synthesis of fatty acidsFASNUpOsteosarcoma[98]LncRNA SNHG16De novo synthesis of fatty acidsSCDUpColorectal cancer[104]LncRNA uc.372De novo synthesis of fatty acidsACC/FASN/SCD1UpLiver cancer[105]LncRNA UPATDe novo synthesis of fatty acidsSCD1UpColon cancer[107]LncRNA uc.372Exogenous uptake of fatty acidsCD36UpLiver cancer[105]LncRNA LNMICCExogenous uptake of fatty acidsFABP5UpCervical cancer[120]LncRNA SNHG7Oxidation of fatty acidsACSL1UpThyroid cancer[130]LncRNA HULCOxidation of fatty acidsACSL1UpHepatocellular carcinoma[132]LncRNA NEAT1Oxidation of fatty acidsACSL4UpProstate cancer[131]LncRNA MACC1-AS1Oxidation of fatty acidsCPT1UpGastric cancer[121]LncRNA HCP5Oxidation of fatty acidsCPT1UpGastric cancer[142]LncRNA AGAP2-AS1Oxidation of fatty acidsCPT1UpBreast cancer[122]LncRNA NEAT1Oxidation of fatty acidsCPT1UpBreast cancer[144]LncRNA SPRY4-IT1Synthesis of triglycerideLipin-2DownMelanoma[150]LncRNA HR1Synthesis of triglycerideSPEBP-1cDownLiver cancer[152]LncRNA NEAT1Degradation of triglycerideATGLUpHepatocellular carcinoma[158]LncRNA Khps1Phospholipid metabolismSphK1UpOsteosarcoma[169]LncRNA HULCPhospholipid metabolismSphK1UpHepatocellular carcinoma[170]LINC00460Phospholipid metabolismSphK1UpColorectal cancer[171]LINC00460Phospholipid metabolismSphK2UpPapillary thyroid carcinoma[172]LINC00520Phospholipid metabolismSphK2UpPapillary thyroid carcinoma[173]LINC00511Phospholipid metabolismPLD1UpCervical cancer[178]LncRNA SLNCR1Phospholipid metabolismPLA2UpNon-small cell lung cancer[181]LncRNA HULCCholesterolFOXM1UpHepatocellular carcinoma[185]LncRNA UCA1Palmitic acidERK/MMP-9UpGastric cancer[190-192]LncRNA ANRILPhospholipid//Large cell lung cancer[193]In addition, lncRNAs can also directly affect the expression of LDLR to promote tumor development. Wang et al. found that lncRNA CASC19 was significantly upregulated in non-small cell lung cancer (NSCLC) and was positively correlated with the proliferation and metastasis of NSCLC cells. It was predicted and proved that LDLR is the downstream target gene of miR-301b-3p in NSCLC, and it is inhibited when interacting with miR-301b-3p. However, CASC19 can sponge miR-301b-3p and restore the effect of LDLR. Therefore, CASC19 plays a role in NSCLC by targeting the miR-301b-3p/LDLR axis to promote the proliferation and metastasis of cancer cells [48].
LncRNAs regulate cholesterol effluxMost peripheral cells and tissues lack the ability to metabolize cholesterol [49]. When the cholesterol in the cell exceeds what the cell needs, cholesterol is either converted to cholesterol ester (CE) by the action of cholesterol acyltransferase (ACAT) [50], which is stored in lipid droplets or secreted in lipoproteins, or is excreted from the cell via an ATP binding cassette (ABC) transporter. Liver X receptors (LXRs) can be used as cholesterol sensors [51]. When intracellular cholesterol is at a high level, LXRs are activated by cholesterol derivative oxysterols [52], and then they can bind to retinoid X receptor alpha (RXRα) as an obligate heterodimerization partner to direct repeat 4 (DR4) in the promoter of ABC transporters [53, 54], thereby activating the expression of ABC transporters to promote cholesterol export.
LXR is composed of alpha and beta subtypes. A number of studies have shown that the activation of LXR can significantly inhibit tumor progression. For example, activating the expression of LXR can inhibit the protein associated with cell proliferation in BC [55]. Activating LXR can block the G1 phase of cancer cells in intestinal tumors and increase caspase-dependent apoptosis to inhibit the development of cancer [56]. In addition, LXR-623, as an activator of LXR, can effectively inhibit the development of glioblastoma (GBM) by activating LXRβ, lowering cholesterol levels, and inducing apoptosis [57]. ATP binding box transporter A1 (ABCA1), a subtype of ABC, can be activated by LXRs to promote cholesterol efflux [58]. When ABCA1 expression is reduced, cholesterol is allowed to accumulate in cells. Cholesterol accumulation leads to reduced membrane fluidity and inhibition of the mitochondrial permeability transition (MPT), which prevents the release of cell death-promoting molecules in mitochondria [59], ultimately promoting the progression of cancer. However, the current research on the regulatory mechanism of lncRNAs on ABC is mostly limited to noncancer diseases. For example, lncRNA GAS5 is highly expressed in atherosclerosis and can recruit enhancer of zeste homolog 2 (EZH2) to the ABCA1 promoter region to promote histone methylation modification of ABCA1. Eventually, transcription of ABCA1 is inhibited, leading to cholesterol accumulation and atherosclerosis [60]. In addition, lncRNA ENST00000602558.1 can directly bind to P65 to promote P65 binding to the promoter of ABCG1 and inhibit the expression of ABCG1 mRNA and protein in vascular smooth muscle cells. The reduction in cholesterol efflux mediated by ABCG1 further leads to dyslipidemia and atherosclerosis [61].
Many lipids are synthesized from FAs, a class of molecules consisting of hydrocarbon chains of varying lengths and degrees of desaturation [6]. Within cells, FAs have various functions, including being a component of the membrane, conducting signals, or being oxidized to release energy. FAs are either obtained from an external source or synthesized from scratch. Under normal conditions, most normal cells preferentially use exogenous FAs to meet their lipid requirements [62]. FAs must be activated by covalent modification of fatty acyl-CoA synthetase (ACS) to synthesize lipids or decompose to produce energy [63]. In cancer, FA metabolism is undoubtedly altered due to the high energy requirements of rapidly proliferating cells [6, 64, 65]. Therefore, the roles of lncRNAs in FA uptake, de novo synthesis, and oxidative degradation in tumor progression are also worthy of study and discussion (Figure 1 and Table 1).
LncRNAs regulate the de novo synthesis of fatty acidsMost normal cells have the ability to take up lipids from the extracellular environment and are thus more inclined to take up FA exogenously[62, 66]. In contrast, an increase in the demand for lipids by tumor cells has been observed in tumors, such as signaling molecules and membrane biosynthesis. Therefore, the de novo synthesis of FA plays a dominant role in tumors [67-69]. The increased demand for FA synthesis becomes an adaptation to the high metabolic demand of cancer cells. The de novo synthesis of FAs requires the action of a variety of enzymes. First, glucose is decomposed into pyruvate and then enters the mitochondria to oxidize and decarboxylate to generate acetyl-CoA; since acetyl-CoA cannot directly penetrate the mitochondrial membrane and needs to synthesize FAs in the cytoplasm, it must first condense with oxaloacetate to form citric acid and then enter the cytoplasm. Then, acetyl-CoA is generated under the action of ATP citrate lyase (ACLY), and finally, saturated FAs are synthesized under the continuous condensation reaction of acetyl-CoA carboxylase (ACC) carboxylation and fatty acid synthase (FASN) [64, 70]. Additionally, saturated FAs can be desaturated by stearoyl-CoA desaturase (SCD) according to cellular requirements to produce monounsaturated FAs [64]. Therefore, inhibiting these enzymes and reducing FA synthesis will effectively limit the growth of cancer cells.
ATP citrate lyaseATP citrate lyase (ACLY) is an enzyme that catalyzes an important step in FA synthesis. It can catalyze the conversion of citric acid and coenzyme A (CoA) to acetyl-CoA and oxaloacetic acid. Acetyl-CoA is essential for FA synthesis and cancer cell proliferation. ACLY is abnormally expressed and active in many tumors [71, 72], such as renal carcinoma [73], pancreatic cancer [74], BC [75] and gastric cancer (GC) [76].
Zheng et al. found that lncRNA TINCR was highly expressed in nasopharyngeal carcinoma (NPC) cells. RNA pull-down assay and RNA-EMSA analysis confirmed that the 1-876 nt region of TINCR can bind ACLY. Subsequently, it was shown that TINCR can inhibit the ubiquitin-mediated degradation of ACLY to protect the proteasome-dependent degradation of ACLY, thereby maintaining the stability of the ACLY protein. The increased stability of the ACLY protein increases the level of acetyl-CoA, hence increasing the content of free fatty acids (FFAs), leading to the increased proliferation, metastasis and chemoresistance of cancer cells [77]. Zhang et al. demonstrated that lncRNA FLJ22763 is downregulated in GC tissue and is closely related to the survival of patients. The researchers revealed a significant negative correlation between FLJ22763 and ACLY mRNAs in GC. After FLJ22763 overexpression, ACLY was significantly downregulated, hence inhibiting GC cell malignancy and xenograft tumor growth [78]. In addition, in recent years, researchers have used the statistical method of weighted correlation network analysis (WGCNA) to determine the coexpression network of lncRNAs and ACLY in GC. WGCNA can identify the genes most related to cancer in the database. Then, a series of methods, including univariate and multivariate Cox regression analyses and Pearson correlation and hypergeometric tests, confirmed that ACLY was correlated with lncRNA PPP1R26-AS1, lncRNA DLEU1 and lncRNA TMPO-AS1 [79]. However, further experiments are needed to verify the relationship between these lncRNAs and ACLY.
In addition, some lncRNAs that regulate the expression of ACLY are also associated with viral infections. Lipid production has a positive effect on virus replication [80]. Linc-Pint is significantly downregulated in HCV-infected hepatocytes. Linc-Pint overexpression may downregulate the protein level of serine/arginine protein-specific kinase 2 (SRPK2) through proteasome degradation [81]. SRPK2 activates serine/arginine (SR) proteins involved in mRNA splicing and maturation [82]. It has been reported that SRPK2 can promote effective splicing by the phosphorylation of SR proteins to increase the stability of ACLY and FASN mRNA [83]. Therefore, the overexpression of Linc-Pint decreased the expression of SRPK2, which further reduce the stability of ACLY and FASN mRNA. Finally, the HCV-induced lipid production pathway is inhibited to limit the replication of HCV [81].
Acetyl-CoA carboxylaseAcetyl-CoA can be carboxylated to malonyl-CoA under the action of acetyl-CoA carboxylase (ACC), which is an indispensable step in FA synthesis [3]. ACC has two different subtypes, ACC1 and ACC2, and the functions of malonyl-CoA produced by them are different. The malonyl-CoA produced under the action of ACC1 mainly promotes the synthesis of FAs, while the malonyl-CoA produced under the action of ACC2 mainly inhibits the oxidation of FAs [84], keeping the FA content in the body at a high level. To further meet the needs of tumor cells, ACC is highly expressed in NSCLC [85], cervical squamous cell carcinoma [86] and colorectal cancer [87].
The mechanisms by which lncRNAs regulate ACC in different tumors are also different. LncRNA DNAJC3-AS1 is highly expressed in colorectal cancer and can regulate the expression of ACC and FASN by activating PI3K/AKT, a recognized oncogenic pathway, thereby promoting tumor progression [87]. Recent studies have demonstrated that lncRNA CTD-2245E15.3 is highly expressed in NSCLC, and its inhibition can regulate lipid metabolism-related genes. Based on this conclusion, the study also showed that the knockout of CTD-2245E15.3 can phosphorylate the Ser117 site of ACC1. The Ser117 site of ACC1 is the inhibitory site of enzyme activity. Its phosphorylation inhibits the enzyme activity of ACC1, which further leads to lipid synthesis obstacles and ultimately inhibits tumor growth. It is concluded that CTD-2245E15.3 promotes the progression of non-small cell lung cancer by regulating the enzyme activity of ACC1 [85].
Fatty acid synthaseFatty acid synthase (FASN) is a multifunctional enzyme that catalyzes the biosynthesis of palmitate esters in an NADPH-dependent manner and thus participates in the synthesis of FAs [88]. FASN is widely expressed in normal cells, and its promotion of expression leads to an increase in FA synthesis. An imbalance in FASN expression can cause many diseases. For example, the high expression of FASN has been demonstrated to be closely related to poor prognosis in various cancers, such as BC [89] and NSCLC [90].
HAGLR is a lncRNA transcribed from the HOXD cluster on human chromosome 2 that is upregulated in multiple cancers, including colon cancer [91], hepatocellular carcinoma [92] and BC [93], and is closely related to progression and unfavorable prognosis. Lu et al. discovered that the level of HAGLR expression in NSCLC increased and was associated with poor prognosis in patients. The level of FASN is positively correlated with the expression of HAGLR. The expression of FASN in NSCLC decreased with the knockdown of HAGLR, which reduced the FFA content in cells and inhibited the proliferation, invasion and tumorigenesis of NSCLC cells [94]. In addition, lncRNA HOTAIR plays a role in regulating chromatin dynamics in gene regulation. As a proto-oncogene, it is highly expressed in a variety of cancers [95]. Knockout of HOTAIR can reduce FASN expression and inhibit FA synthesis in NPC cells, thereby inhibiting their proliferation and invasion [96]. It is worth mentioning that matrix metalloproteinase-9 (MMP-9), as a potential cancer marker [97], has been downregulated in the knockout of HAGLR and HOTAIR [94, 96]. Therefore, whether FASN affects MMP-9 plays a role and needs further verification. Moreover, Zhou et al. found that lncRNA PVT1 is overexpressed in osteosarcoma, reducing the survival rate of patients with osteosarcoma. PVT1 mainly acts as a competitive endogenous RNA (ceRNA) to negatively regulate miR-195 in osteosarcoma cells to enhance FASN expression, thereby promoting osteosarcoma cell migration and invasion [98].
Stearoyl-CoA desaturaseSCD is a major enzyme involved in the synthesis of monounsaturated FAs. Two types of SCD isoforms, SCD1 and SCD5, have been found in humans [99]. Among them, SCD1 is widely expressed in tissues. The ratio between saturated FAs and unsaturated FAs is very important for cancer cells, and its changes will affect cell fluidity and protein dynamics [84]. The increase in unsaturated FAs not only facilitates the proliferation and metastasis of cancer cells but also inhibits cell apoptosis. In many types of cancers, increased expression of SCD1 can lead to the proliferation and invasion of cancer cells, and inhibition of SCD1 expression can inhibit tumor progression in vivo, such as prostate cancer [100], bladder cancer [101], lung cancer [102] and clear cell kidney cell carcinoma [103].
The expression of lncRNA SNHG16 is upregulated in colorectal cancer and is regulated by c-Myc. The upregulation of SNHG16 was confirmed after c-Myc overexpression. To further clarify the potential molecular mechanism of SNHG16, genome-wide transcription profiling was performed after knocking down SNHG16. Next, Ingenuity Pathway Analysis (IPA) was used to determine that knocking out SNHG16 can affect the expression of lipid metabolism-related genes. The study discovered that the miRNA bound by SNHG16 cotargeted the 3'-UTR of SCD mRNA. Therefore, SNHG16 may upregulate the expression of SCD through a ceRNA mechanism to promote the proliferation and migration of colorectal cancer cells [104]. In addition, UC (ultraconserved, UC) RNA is also a long noncoding RNA. After overexpression of uc.372 in liver cancer HepG2 cells, FASN, ACC, SCD1 and CD36 were all upregulated. To further study the molecular mechanism of uc.372 regulating the expression of ACC, FAS, SCD1 and CD36. Microarray analysis showed that pri-miR-195 and pri-miR-4668 were complementary to the ultraconserved region of uc.372. Overexpression of uc.372 can inhibit the maturation of miR-195 and miR-4668 by specifically binding to pri-miR-195 and pri-miR-4668 [105]. Based on previous research, ACC and FAS are the target genes of miR-195 [106]. Experimental prediction and verification proved that SCD1 and CD36 are the target genes of miR-4668 [105]. The overexpression of uc.372 reduced the suppression of ACC and the expression of FASN by miR-195 and the suppression of SCD1 and the expression of CD36 by miR4668. Therefore, it can be concluded that uc.372 can upregulate ACC, FASN, SCD1 and CD36 through the pri-miR-195/miR-195 and pri-miR-4668/miR-4668 signal axes to drive fat accumulation in HepG2 cells [105]. Moreover, lncRNA UPAT is necessary for the tumorigenicity of colon cancer cells. Through a series of experiments to identify proteins that may be related to UPAT, researchers found that UPAT can interfere with the ubiquitination and degradation of UHRF1 in colon tumors through the proteasome, thereby maintaining the stability of UHRF1. Knockout of UPAT or UHRF1 reduces the expression of SCD1, but UHRF1 has nothing to do with the SCD1 promoter region. Therefore, it is speculated that SCD1 is not a direct target of UHRF1 but is indirectly upregulated downstream of UHRF1 and UPAT. The effect of lncRNA UPAT on SCD1 needs further study [107].
LncRNA regulates the exogenous uptake of fatty acidsAlthough the de novo synthesis of FA in cancer occupies a major position in the synthesis of macromolecules, some FAs can also be transported by certain proteins [64], and circulating FAs can be absorbed and utilized to provide support for the survival and development of cancer cells [84]. Therefore, transport proteins such as fatty acid translocase (FAT/CD36) and fatty acid-binding protein (FATP) in the FA uptake pathway may become potential targets for cancer treatment.
Fatty acid translocaseFAT/CD36 is a widely expressed transmembrane protein that mediates the uptake of FAs. It can also bind to carnitine palmityl transferase 1 (CPT1) in fatty acid oxidation (FAO), thus promoting FAO and providing sufficient energy for the rapid proliferation and development of cancer cells [108]. However, few studies have demonstrated the influence of noncoding RNAs on FAT/CD36 regulation in cancer. Only studies have shown the effect of noncoding RNAs on FAT/CD36 in atherosclerosis.
CD36 is a key mediator of macrophage phagocytosis of oxidized low-density lipoprotein (oxLDL) in atherosclerosis [109]. In recent years, studies have found that macrophages can change their phenotypes according to changes in the microenvironment and thus have diverse functions. The two main macrophage phenotypes are classically activated macrophages (M1) and selectively activated macrophages (M2) [110]. The M1 phenotype has obvious proinflammatory and antitumor activities, while the M2 phenotype is an anti-inflammatory phenotype with protumor activity [111]. Although both M1 and M2 macrophages are present in atherosclerosis, M1 macrophages are a dominant phenotype associated with plaque progression [112]. M1 macrophages form foam cells after phagocytosing oxLDL. Foam cells then secrete proinflammatory mediators to further aggravate the production of unstable atherosclerotic plaques [113]. LncRNA MALAT1 can accumulate β-catenin on the CD36 promoter-binding site in macrophages, promote CD36 transcription and lipid uptake [114], and ultimately accelerate the occurrence of atherosclerosis. LncRNA PELATON is rich in unstable atherosclerosis, especially in the areas where the nuclei of macrophages gather. RNA sequencing found that there was a strong positive correlation between PELATON and CD36. With the decrease in PELATON, CD36 expression was significantly reduced, leading to marked reductions in macrophage phagocytosis and lipid absorption, which inhibited plaque progression [115]. Apart from atherosclerosis, macrophages are also closely related to the poor prognosis of tumors [116]. Tumor-associated macrophages (TAMs) are the main component of inflammatory cells that infiltrate cancer. In advanced cancers, most macrophages are of the M2 phenotype, which highly stimulates tumor progression [116]. Therefore, whether lncRNAs affect the role of TAMs by regulating CD36 still needs to be studied.
Fatty acid-binding proteinFatty acid-binding protein (FABP) is involved in FA transport and metabolism, and FABP can bind to FFAs and transport them to various organelles for further oxidation or esterification. Multiple studies have shown that FABP5 can be involved in the occurrence and development of hepatocellular carcinoma (HCC) [117], BC [118] and prostatic carcinomas [119].
Shang et al. found that lncRNA LNMICC was a valuable prognostic predictor of cervical cancer. Multiple regression analysis revealed that high LNMICC expression was significantly related to the BMI of cervical cancer patients. Therefore, it is speculated that LNMICC is related to the reprogramming of FA metabolism in cervical cancer. For further confirmation, the levels of cell-related lipids and the expression of key FA metabolism were measured at different expression levels of LNMICC. Researchers have revealed that the levels of cell-related lipids and the expression of key enzymes for FA metabolism in cervical cancers with different LNMICC levels are different. Next, the researchers further studied how LNMICC exerts its biological function to reprogram FA metabolism. Based on bioinformatics analysis, LNMICC is located upstream of the FABP5 gene, and the mRNA levels of LNMICC and FABP5 are positively correlated. A series of experiments discovered that LNMICC can target the FABP5 promoter region by recruiting NPM1 to interact directly with LNMICC, thereby enhancing the transcription of FABP5. Finally, combined in vivo and in vitro biological function experiments show that LNMICC can promote lymph node metastasis and epithelial-mesenchymal transition (EMT) by directly regulating FABP5-mediated FA metabolic reprogramming [120].
LncRNAs regulate the oxidation of fatty acidsFatty acid oxidation (FAO) is an important catabolic process in which living organisms use FAs as energy sources. FAs must be activated before oxidation. FAs are activated under the action of ACS to generate acyl-CoA. Acyl-CoA enters the mitochondria under the action of carnitine palmityl transferase (CPT) and is oxidized and decomposed to produce acetyl-CoA, FADH2 and NADH. Acetyl-CoA enters the TCA cycle and is completely oxidized to produce ATP, while NADH and FADH2 enter the electron transport chain to produce ATP. According to reports, many types of cancers show high FAO activity, such as GC [121], BC [122], glioma [123] and acute myeloid leukemia (AML) [124]. FAO can promote tumor development by increasing the production of ATP. NADPH produced by acetyl-CoA in the TCA cycle provides cancer cells with a redox ability to resist oxidative stress [125]. Therefore, targeting key enzymes in FAO may hopefully provide an effective method for cancer treatment.
Acyl-CoA synthetase long chain family memberFAs can be catabolized to acetyl-CoA to promote the production of ATP or serve as the raw material for the synthesis of TAG, PL and CE [63, 126]. However, these two distinct pathways require a common initial step, which is referred to as FA activation. ACS is an enzyme necessary for the activation of FAs. Among them, acyl-CoA synthetase long-chain family members (ACSLs) are the most critical enzymes responsible for the activation of the most abundant long-chain FA metabolism in mammalian cells. ACSLs include ACSL1, ACSL3, ACSL4, ACSL5 and ACSL6 [127]. Different members of ACSLs play various roles in cancer, and their expression varies in different cancers. For example, ACSL4 is highly expressed in colon adenocarcinoma [128], but it inhibits tumors in GC [129].
According to reports, lncRNAs also have the potential to regulate ACSLs. For example, lncRNA SNHG7 plays a carcinogenic effect in thyroid cancer (TC). After SNHG7 is knocked down, the mRNA and protein levels of ACSL1 are suppressed. Cell proliferation and migration experiments revealed that the increase in ACSL1 can reverse the inhibitory effects of SNHG7 on cell proliferation and migration when SNHG7 is depleted. In addition, bioinformatics analysis found that miR-449a can act as a miRNA that simultaneously binds SNHG7 and ACSL1. To analyze the role of miR-499a in the regulation of ACSL1 by SNHG7, researchers conducted RIP and luciferase reporter gene analyses. The experiment concluded that SNHG7 can be used as a sponge for miR-449a, thereby increasing ACSL1 in TC cells and promoting the proliferation and migration of TC cells [130]. Additionally, lncRNA NEAT1 is highly expressed in docetaxel-resistant prostate cancer patients and cell lines. The CCK-8 experiment confirmed that after knocking out NEAT1, the IC50 value of docetaxel on prostate cancer cells decreased significantly. Compared with docetaxel-treated parental cells, NEAT1 knockdown promoted the sensitivity of cells to docetaxel and reduced cell proliferation and invasion. To study the potential molecular mechanism of NEAT1 in prostate cancer, bioinformatics predictions and experiments proved that miR-34a-5p and miR-204-5p are potential targets of NEAT1 and are inhibited by NEAT1. Next, the downstream targets of miR-34a-5p and miR-204-5p were predicted and verified to be ACSL4. miR-34a-5p and miR-204-5p can inhibit the expression of ACSL4 by targeting the 3'-UTR of ACSL4 and then reduce the ability of prostate cancer cells to be resistant to docetaxel. In summary, NEAT1 can enhance the expression of ACSL4 by sponging miR-34a-5p and miR-204-5p, thereby promoting docetaxel resistance in prostate cancer cells and accelerating the progression of prostate cancer [131]. In addition, studies have shown that lncRNA HULC can regulate abnormal lipid metabolism in the body through the miR-9/PPARA/ACSL1/signaling pathway [132], increase the accumulation of TG and CE in HCC tissues, and promote the growth and development of HCC. The mechanism of this signaling pathway will be explained in detail below.
Carnitine palmityl transferaseAt present, it is known that FAO plays a role in inducing cancer cell metastasis and chemotherapy resistance and improving the stemness of cancer cells [65, 121, 133-135]. Among them, CPT1, as the rate-limiting enzyme of FAO, provides the first and rate-limiting steps of FA transport to mitochondria for oxidation. CPT1 can directly control the production of ATP and NADPH in the FAO process, which constitutes an important part of cancer metabolic adaptation [125]. Therefore, fully exploring the molecular mechanism of lncRNA regulating CPTI can identify a new therapeutic window in cancer treatment intervention.
Metastasis-associated in colon cancer-1 (MACC1) is a transcriptional regulator of EMT. MACC1 is upregulated in various tumors and enhances cell proliferation, invasion and chemotherapy resistance [136-140]. MACC1-AS1 is the cognate antisense lncRNA of MACC1. Recent studies have shown that MACC1-AS1 can be induced to be expressed by mesenchymal stem cells (MSCs) [121]. In previous reports, MSCs secreted TGF-β1, a key cytokine. TGF-β exerts cellular effects by binding to TGFβ receptors (TGFβR-I and TGFβR-II) and then activates the downstream SMAD family [141]. In a recent study, it was found that when MSCs are cocultured with GC cell lines, TGF-β1 secreted by MSCs can be directly combined with TGFβR-I and TGFβR-II in GC cells to activate SMAD2 and SMAD3 and then induce the upregulation of MACC1-AS1 in GC cells. The upregulation of MACC1-AS1 activates the FAO pathway in GC cells, and the expression levels of FAO-related enzymes (CPT1 and ACS) are significantly increased, which promotes stemness and chemoresistance in GC. In addition, to clarify the potential mechanism of MACC1-AS1 on FAO-dependent dryness and chemical resistance, it was found that miR-145-5p is located downstream of MACC1-AS1 based on the prediction of the LncRNASNP database and experimental verification, and MACC1-AS1 can directly bind to miR-145-5p and inhibit the expression of miR-145-5p. miR-145-5p partially reversed the promoting effect of MACC1-AS1 on the expression of CPT1 and stem genes and inhibited the occurrence of drug resistance and FAO [121]. In summary, the TGF-β1/MACC1-AS1/miR-145-5p/CPT1 signaling axis contributes to FAO-dependent stem and chemoresistance, indicating that targeting this signaling pathway may be a potential strategy to inhibit stem cells and chemoresistance induced by MSCs. LncRNAs termed HCP5 could be induced in GC cells by coculture with MSCs and were reported to play an important role in elevating GC cancer cell stem properties and chemoresistance and in predicting a poor prognosis. The pull-down assay showed that miR-3619-5p can be pulled down by HCP5 in GC cells, indicating that there is an interaction between HCP5 and miR-3619-5p. Then, in the biological function experiment, the effects of HCP5 and miR-3619-5p were opposite. HCP5 can exert a sponge effect on miR-3619-5p to promote the activity of CPT1 in GC cells. To further explore the molecular mechanism of this, KEGG analysis revealed that miR-3619-5p was significantly related to the AMPK pathway. The most significantly downregulated gene after miR-3619-5p overexpression was PPARG coactivator 1α (PPARGC1A), a key regulator of the AMPK pathway [142]. It was previously reported that PGC1α, the protein product of PPARGC1A, is a transcriptional coactivator responsible for lipid metabolism [143]. This study revealed that PGC1α can form a transcription complex with CEBPB to activate the transcription of CPT1 and ultimately promote FAO in GC cells. Therefore, the FAO of GC cells can be driven by the HCP5/miR-3619-5p/PPARGC1A/PGC1α/CPT1 axis in GC cells activated by MSCs and ultimately promote the chemoresistance and stemness of GC cells [142].
In addition, another lncRNA induced by MSCs is lncRNA AGAP2-AS1, which was significantly upregulated in BC cells cocultured with MSCs. Bioinformatics analysis showed that the target gene of AGAP2-AS1 was enriched in FAO metabolism. Then, experiments verified that AGAP2-AS1 mediated stemness and trastuzumab resistance by targeting CPT1 and ACS. Further research on the regulatory mechanism of AGAP2-AS1 found that the interaction between AGAP2-AS1 and CPT1 is achieved in two ways. First, AGAP2-AS1 can interact with Hur to produce AGAP2-AS1-Hur and then directly bind to CPT1 mRNA to improve the stability of CPT1. Second, AGAP2-AS1 inhibited the expression of miR-15a-5p through sponge action to increase CPT1 mRNA. Under the combined actions of these two pathways, AGAP2-AS1 can upregulate the expression of CPT1 at the mRNA level, and the FAO of BC cells can be promoted, thereby mediating the characteristics of cancer stem cells and the resistance of trastuzumab [122]. In addition, it has been reported that lncRNA NEAT1 can competitively bind to miR-107 in BC cells, indirectly inhibit the inhibitory effect of miR-107 on CPT1, promote FA oxidation, and provide ATP to regulate the growth and metastasis of BC [144].
TG is a key energy source composed of FFA. When there is too much FA, most of the FA can be connected to the glycerol backbone to form TG, which is then stored in cytoplasmic lipid droplets (LDs) [145]. This storage itself is not disease-causing, but TG, as a substance for storing FA, can release FA at any time. This ready availability may provide cancer with much energy and promote tumor proliferation and metastasis [64]. The accumulation of lipids can also cause lipotoxicity, inducing apoptosis [146]. Therefore, increasing FA storage and inhibiting FA release may also be cancer suppression strategies [147] (Figure 1 and Table 1).
LncRNAs regulate the synthesis of triglycerideFirst, FA is activated into fatty acyl-CoA (FA-CoA), and FA-CoA forms lysophosphatidic acid (LPA) under the action of glycerol 3-phosphate acyltransferase (GPAT). LPA is converted into phosphatidic acid (PA) under the action of acylglycerophosphate acyltransferase (AGPAT). Then, phosphatidic acid phosphohydrolase (PAP or lipin) removes the phosphate group from PA to form diacylglycerol (DG). Finally, diacylglycerol acyltransferase (DGAT) esterifies DG and FA-CoA to TG [148, 149]. Therefore, by reducing FA utilization and increasing its storage as TG, tumor progression can be inhibited. For instance, lncRNA SPRY4-IT1 is highly expressed in melanoma, and the protein related to SPRY4-IT1 is identified as lipin-2 by mass spectrometry analysis. Lipin-2 can be used as the direct target of lncRNA SPRY4-IT1. Downregulation of SPRY4-IT1 resulted in the enhancement of lipin-2 mRNA, protein and enzyme activities. Due to the effective conversion of DAG mediated by lipin-2 to TAG, both the DGAT mRNA expression level and the TG content increased. Based on these results, it is speculated that SPRY4-IT1 knockdown may induce the apoptosis of melanoma cells through lipin-2-mediated lipotoxicity, but this speculation needs further experimental verification [150].
In addition to directly regulating the enzymes in the TG synthesis pathway, lncRNAs can also indirectly regulate the synthesis of FA and TG by regulating SREBP-1c to achieve lipid metabolism reprogramming. SREBP-1c mainly activates the transcription of multiple genes, such as ACLY, ACC, FAS, SCD-1, and GPAT. The enzymes encoded by these genes can participate in the synthesis of FAs and TG [151]. Therefore, targeting SREBP-1c can effectively inhibit the production of lipids and prevent the proliferation of cancer cells. Li et al. found that overexpression of lncRNA HR1 in Huh7 liver cancer cells can inhibit the phosphorylation of AKT. AKT acts as an upstream regulator of Forkhead Box O1 (FoxO1), and inhibition of AKT phosphorylation reduces the nuclear translocation of FoxO1, which causes a large accumulation of FoxO1 in the nucleus [152]. In previous reports, FoxO1 was generally regarded as a tumor suppressor that can antagonize the combination of the LXRα/RXR heterodimer and LXR elements (LXREs) in the promoter region of SPEBP-1c, thereby downregulating the transcription of SREBP-1c [153]. In summary, lncHR1 may inhibit the accumulation of TG in liver cancer cells through the AKT/FoxO1/SREBP-1c pathway and ultimately may reduce the energy supply of cancer cells to inhibit tumor progression [152].
LncRNA regulates the degradation of triglycerideFA can be stored in the form of TG. When needed, each TG molecule can be sequentially catalyzed by triglyceride lipase (ATGL), hormone-sensitive lipase (HSL) and monoacylglycerol lipase (MAGL) to release three FAs. ATGL is usually considered the key enzyme for the release of FAs from TG stores [154, 155]. High levels of ATGL have been discovered in a variety of cancers, such as BC [156] and lung cancer [157]. Liu et al. found that the expression of ATGL in HCC tissues is upregulated, which is related to poor prognosis. Co-lncRNA software screening and assay identification showed that lncRNA NEAT1 and ATGL were positively correlated in HCC tissues. To explore the molecular mechanism of NEAT1/ATGL, researchers used a series of bioinformatics methods to determine whether there is a potential miRNA to regulate ATGL. Studies have found that miR-124-3p can directly bind ATGL to inhibit the expression of ATGL. However, the interaction between NEAT1 and ATGL may occupy the binding site of miRNA-124-3p and ATGL, weakening the inhibitory effect of miR-124-3p on ATGL [158]. In addition, it has been proven that FA is the main physiological ligand that activates the known oncogene PPARα in liver cancer [159]. NEAT1 abnormally regulates lipolysis, which leads to an increase in FA, which eventually increases the expression of PPARα, thereby promoting the growth and reproduction of HCC cells [158]. These results indicate that NEAT1 can mediate the growth of HCC cells through miR-124-3p/ATGL/DAG + FA/PPARα signaling.
Phospholipids are the key components of cell membranes and can be divided into two categories: glycerophospholipids and sphingomyelin. Phospholipids play important roles in cells, such as chemical energy storage, cell signal transmission, and cell-cell interactions [160]. Since uncontrolled cell proliferation in cancer requires sufficient energy and cell structural units, the phospholipids in cancer cells need to be actively biosynthesized so that phospholipids can perform the abovementioned functions and participate in the development of cancer (Figure 1 and Table 1).
Sphingosine kinasesSphingosine kinases (SphKs) are key regulatory enzymes that catalyze the formation of sphingosine-1-phosphate (S1P). SphK1 is a cytoplasmic protein. Once Sphk1 is activated by various extracellular signal transduction pathways, it is phosphorylated by ERK1/2 and transferred to the plasma membrane to phosphorylate sphingosine to form S1P [161]. Conversely, SphK2 is mainly located in the nucleus and part of the mitochondria, and S1P can also be produced at these sites [162, 163]. Currently, S1P is involved in regulating cell growth, proliferation, survival, migration and angiogenesis [164]. Therefore, SphKs may be an important tumorigenesis regulator. Further understanding of the molecular mechanisms regulating SphK expression may reveal new directions for therapeutic strategies. It has been found that the abnormal expression of SphKs is associated with the development and poor prognosis of many cancers [165-168]. There are a variety of lncRNAs that can participate in various types of cancer-related biological abnormalities by regulating the expression of SphKs.
In osteosarcoma, Anna et al. found that lncRNA Khps1 is an antisense RNA that can activate the transcription of SphK1. Therefore, researchers have studied the molecular mechanism by which Khps1 regulates the expression of SphK1. SphK1 contains two putative triplex-forming regions (TFRs) upstream of the transcription start site of its subtype B (SphK1-B). Khps1 can directly interact with TFR2 to form a purine-rich RNA-DNA triplet through Hoogsteen hydrogen bond base pairing, which is anchored to the SphK1-B promoter. Khps1 linked to SphK1-B can interact with histone acetyltransferase p300/CBP, which modifies histone acetylation to create an open chromatin structure. The change in chromatin structure promotes the binding of the SphK1-B upstream transcription factor E2F1 to the SphK1-B promoter to activate SphK1 transcription. Interestingly, the DNA fragment containing the Khps1 promoter also has a binding site for the transcription factor E2F1. Combined with kinetic data analysis, E2F1 induces Khps1 transcription before promoting SphK1-B mRNA upregulation. Therefore, elevated levels of E2F1 will induce the transcription of Khps1. Then, Khps1 changes the chromatin structure of SphK1-B to promote the combination of E2F1 and the SphK1-B promoter to activate SphK1-B transcription and ultimately promote the proliferation of osteosarcoma cells and limit cell apoptosis [169]. In HCC, Zhan et al. found that lncRNA HULC can increase the expression levels of SphK1 mRNA and protein a
留言 (0)