Diabetic peripheral neuropathy (DPN) affects at least 50% of people with diabetes and is associated with pain, numbness, loss of sensation, and diabetic foot ulceration (Pop-Busui et al., 2017). Multiple risk factors, including hyperglycemia, hypertension, hyperlipidemia, increased weight, and reduced physical activity, play a significant role in the development and progression of DPN. The identification of patients at the highest risk for the development and progression of DPN would enable the selection of patients for more aggressive risk factor reduction and allow the identification of individuals who may benefit the most in clinical trials of disease-modifying therapies.
Symptoms and signs, as well as quantitative sensory tests, are highly variable (Werner et al., 2013). While nerve conduction studies (Mallik and Weir, 2005) and skin biopsies are sensitive measures of nerve damage, they require expertise to acquire and interpret and have limited utility, especially over time (Guldiken et al., 2023). Corneal confocal microscopy (CCM), a rapid, non-invasive measure of small nerve fiber damage and repair, has emerged as a predictive biomarker for the development of DPN (Lewis et al., 2020; Perkins et al., 2021). Studies have established the predictive accuracy of CCM for incident DPN (Pritchard et al., 2015; Perkins et al., 2021). However, relying on a single-time-point measurement of corneal nerve fiber length (CNFL) may have limitations as the trajectory of DPN and the change in corneal nerve morphology are not linear and are influenced by multiple risk factors such as HbA1c, lipid profile, body weight, physical activity, and ongoing treatment (Ponirakis et al., 2020, 2022).
This prospective longitudinal study investigated whether sustained corneal nerve damage is associated with incident DPN and the progression of neuropathic symptoms and deficits in individuals with type 2 diabetes (T2D). It also evaluated the predictive ability of single-time-point baseline CCM measurements and sustained abnormal CCM measures for DPN onset.
2 Materials and methods 2.1 Project designParticipants with type 2 diabetes (T2D) and healthy controls aged between 18 and 80 years were recruited from the National Diabetes Center in Hamad General Hospital in Qatar between January 2017 and October 2022. The study obtained ethics approval from the WCM-Q IRB (#14–00058 and 20–00024) and HMC IRB (#15103/15 and MRC-01-21-386) and adhered to the principles of the Declaration of Helsinki. All participants provided informed consent prior to enrollment.
The exclusion criteria for all participants included a history of allergy to oxybuprocaine, a local anesthetic used for the CCM, severe chronic dry eyes and corneal dystrophies, and a history of ocular injury or surgery in the preceding 12 months. The exclusion criteria also included chronic kidney disease (CKD) stages 4 and 5, medication leading to insulin resistance (e.g., corticosteroids), pregnancy, active retinopathy, and any cause of neuropathy other than diabetes, including Sjogren’s syndrome, systemic lupus erythematosus, human immunodeficiency virus (HIV), hepatitis B and C, inherited neuropathies, tumors, and alcohol excess. Healthy controls did not have chronic dry eyes, corneal dystrophies, a history of ocular injury or surgery in the preceding 12 months, or other causes of neuropathy, such as diabetes, Sjogren’s syndrome, systemic lupus erythematosus, HIV, hepatitis B and C, inherited neuropathies, tumors, or excessive alcohol consumption.
Participants with T2D were assessed at baseline and years 1, 2, and between 4 and 7 years. Healthy controls were enrolled only for the baseline visit to define the cutoff values for abnormal corneal nerve morphology.
2.2 Independent variablesCorneal nerve morphology was quantified using the Heidelberg Retina Tomograph and Rostock Cornea Module (Heidelberg Engineering GmbH, Heidelberg, Germany) (Petropoulos et al., 2013). The participants’ eyes were anesthetized using a drop of oxybuprocaine hydrochloride 0.4% (Chauvin Pharmaceuticals, Chefaro, United Kingdom). Viscotears gel (Carbomer 980, 0.2%, Novartis, UK) was applied on the front of the eye as the coupling agent between the cornea and the cap on the CCM. The participant was instructed to fixate on a target with the eye not being examined. Several scans of the sub-basal nerve plexus in the central cornea were captured per eye for ~2 min. The field of view of each image is 400×400 μm. At a separate time, three high-clarity images per eye of the sub-basal nerve plexus were selected by one researcher blind to the participant’s health condition. Criteria for image selection included depth, focus position, and contrast (Kalteniece et al., 2017). CNFD (number of main nerve fibers/mm2), CNBD (number of branches/mm2), and CNFL (length of main nerves and branches mm/mm2) were manually quantified using CCMetrics (Dabbah et al., 2011).
Abnormal CNFD, CNBD, and CNFL were based on a cutoff value of 1.5 standard deviations (SDs) below the mean of age-matched healthy controls and were considered sustained when persisting for ≥50% of the study duration.
2.3 Dependent variablesNeuropathic symptoms were assessed using the Douleur Neuropathique en 4 (DN4) questionnaire (Spallone et al., 2012). The DN4 comprises questions related to neuropathic symptoms: burning, painful cold, electric shocks, tingling, pins and needles, numbness, and itching.
Vibration perception threshold (VPT) was measured on the pulp of the large toe with a Neurothesiometer (Horwell, Scientific Laboratory Supplies, Wilford, Nottingham, United Kingdom). The test was repeated three times, and the average value was recorded.
Sudomotor nerve function was quantified by evaluating electrochemical skin conductance (ESC) using Sudoscan (Impeto Medical SAS) (Selvarajah et al., 2015). Sudoscan evaluates sympathetic innervation of the sweat gland based on sweat chloride concentrations in response to the voltage applied and is reported as ESC in microsiemens (μS).
The diagnosis of DPN was based on either ≥4 neuropathic symptoms and impaired VPT ≥15 V in the feet or ≥ 4 neuropathic symptoms and abnormal CNFL ≤17 mm/mm2.
2.4 CovariatesClinical and metabolic measures, including age, diabetes duration, body mass index (BMI), systolic (SBP) and diastolic (DBP) blood pressure, HbA1c, and lipid profile, were recorded from the electronic medical record system.
2.5 Data analysis and statisticsThis is the first prospective study investigating whether sustained abnormal CCM measures are associated with the development of DPN; therefore, no power calculation was determined.
Numeric variables and frequency distributions for categorical variables were summarized as mean ± standard deviation or n (%). Continuous and categorical variables between the groups with sustained and non-sustained abnormal corneal nerve measures were compared using the unpaired t-test and chi-square, respectively.
A bivariate linear regression analysis was performed with VPT, ΔVPT, DN4 score, ΔDN4 score, ESC, and ΔESC as dependent variables, sustained abnormal CNFD, CNBD, and CNFL as independent variables, and age, diabetes duration, blood pressure, body weight, BMI, HbA1c, and lipid profile as confounders. All dependent variables were normally distributed, as assessed by Q–Q plots and histograms. Dependent variables that were significant at the bivariate level were included in the multiple linear regression analysis and presented as the regression coefficient (95% CI).
A binary logistic regression analysis was performed with the development of DPN and burning pain and/or numbness as dependent variables, sustained abnormal CNFD, CNBD, and CNFL as independent variables, and age, diabetes duration, poor glycemic control, hyperlipidemia, hypertension, and obesity as confounders. Variables with a p-value of ≤0.05 at the bivariate level were included in the multiple logistic regression. Adjusted odds ratios (AORs), 95% confidence intervals (CIs), and p-values are presented.
A receiver operating characteristic (ROC) curve analysis was used to determine the predictive utility of abnormal baseline and sustained abnormal CCM measures for the onset of DPN. The area under the curve (AUC), 95% CI, and p-value were calculated to quantify the effectiveness of these measures in discriminating between participants with and without the development of DPN during the study.
All statistical calculations were performed using IBM-SPSS version 26 (SPSS Inc., Armonk, NY). A two-tailed p-value of ≤0.05 was considered significant.
3 ResultsOf the 134 participants with T2D enrolled, 107 (79.9%) aged 54.8 ± 8.5 years old, with a mean duration of T2D of 13.3 ± 7.3 years, underwent follow-up assessments. Of the 107 participants, 29 (27.1%) completed 1 follow-up assessment, 38 (35.5%) completed 2 follow-up assessments, and 40 (37.4%) completed all 3 follow-up assessments. The median duration of follow-up assessments was 4 years, ranging from 1 to 7 years.
The cutoff values for abnormal CNFD, CNBD, and CNFL based on 1.5 standard deviations below the mean of 57 closely age-matched healthy controls (50.6 ± 16.9 vs. 54.8 ± 8.5 years, p = 0.11) were ≤ 24 fibers/mm2, ≤21 branches/mm2, and ≤ 16 mm/mm2, respectively.
Over a median duration of 4 years, sustained abnormal CNFD, CNBD, and CNFL were present in 51 (48%), 23 (22%), and 63 (59%) of the T2D cohort, respectively. Within the remaining cohort, the majority had normal corneal nerve morphology (85.2–91.1%) throughout the study duration, while a proportion had abnormal corneal nerve morphology on one occasion only (temporary) (8.9–14.8%).
Corneal nerve measures differed significantly between those with normal, temporary, and sustained abnormality in corneal nerve morphology: CNFD 32.3 ± 6.6 fibers/mm2 vs. 28.2 ± 3.5 fibers/mm2 vs. 21.3 ± 7.6 fibers/mm2 (p < 0.0001); CNBD 78.8 ± 31.4 branches/mm2 vs. 61.8 ± 41.4 branches/mm2 vs. 32.0 ± 28.6 branches/mm2 (p < 0.0001); and CNFL 22.7 ± 4.0 mm/mm2 vs. 20.2 ± 2.2 mm/mm2 vs. 14.4 ± 5.2 mm/mm2 (p < 0.0001), respectively (Figure 1). For the purposes of this study, we assessed the differences between those with and without sustained abnormal corneal nerve morphology, with the latter including those with normal and temporary abnormal corneal nerve morphology.
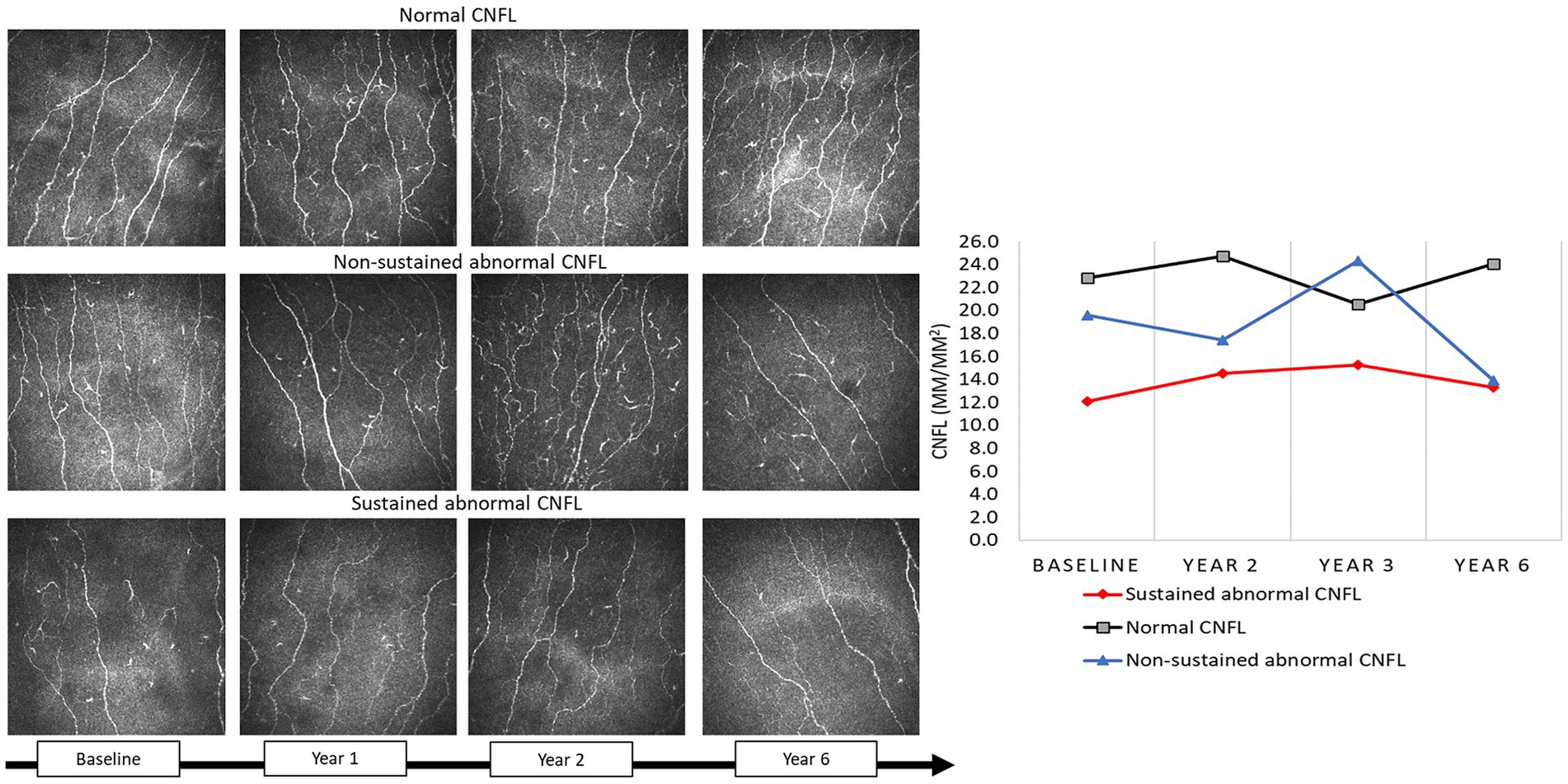
Figure 1. CCM images in a participant with type 2 diabetes with normal CNFL, non-sustained abnormal CNFL, and sustained abnormal CNFL at baseline, 1, 2, and year 6.
The prevalence of DPN at baseline was 18/107 (16.8%). Of the 89 participants without DPN at baseline, 13 (14.6%) developed DPN over a median duration of 4 years (1–7 years range).
3.1 Association of sustained abnormal corneal nerve morphology with clinical characteristicsParticipants with sustained abnormalities in CNFD (n = 51) were significantly older (57.2 ± 6.4 vs. 52.6 ± 9.6, p < 0.01) compared to those without sustained abnormalities in CNFD (n = 56) (Table 1). Those with a sustained abnormality in CNBD (n = 23) had a higher baseline DBP (p = 0.01) compared to those without sustained abnormality in CNBD (n = 84). Diabetes duration, HbA1c, total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), SBP, body weight, and BMI were comparable between the groups with and without sustained abnormal corneal nerve morphology.
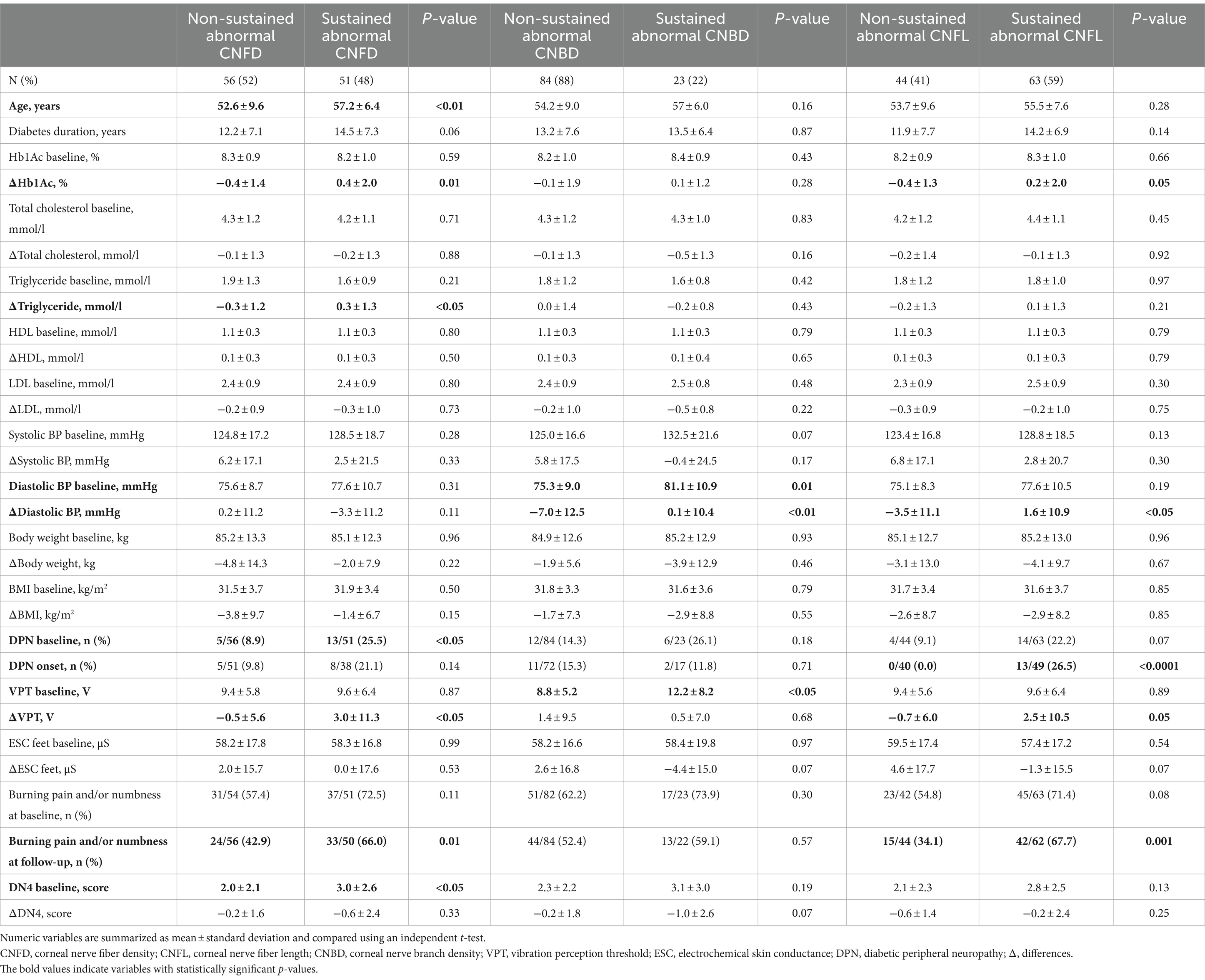
Table 1. Comparison of clinical and neuropathic measures in patients with type 2 diabetes at baseline and their change between sustained and non-sustained abnormal corneal nerve measures.
In those with sustained abnormal CNFD, there was a progressive increase in ΔHbA1c (0.4 ± 2.0% vs. 0.4 ± 1.4%, p = 0.01) and triglycerides (0.3 ± 1.3 mmoL/L vs. −0.3 ± 1.2 mmoL/L, p < 0.05) compared to those without sustained abnormal CNFD. In those with sustained abnormal CNBD or CNFL, there was a progressive increase in Δdiastolic BP (0.1 ± 10.4 vs. −7.0 ± 12.5, p < 0.01, and 1.6 ± 10.9 vs. −3.5 ± 11.1, p < 0.05).
3.2 Association of sustained abnormal corneal nerve morphology with neuropathy progressionAt baseline, sustained abnormal CNFD was associated with a higher prevalence of DPN (25.5% vs. 8.9%, p < 0.05) and neuropathic symptoms (DN4 score: 3.0 ± 2.6 vs. 2.0 ± 2.1, p < 0.05). Sustained abnormal CNBD was associated with higher VPT (12.2 ± 8.2 vs. 8.8 ± 5.2, p < 0.05). There was no association between abnormal corneal nerve morphology and sudomotor function (p = 0.54–0.99).
At follow-up, sustained abnormal CNFD was associated with a progressive increase in vibration perception ΔVPT (3.0 ± 11.3 vs. −0.5 ± 5.6, p < 0.05) and a higher percentage of patients with burning pain, numbness, or both (66.0% vs. 42.9%, p = 0.01). The decrease in vibration perception was associated with diabetes duration (p = 0.001), weight loss (p = 0.01), increased triglycerides (p < 0.0001), and decreased HDL (p = 0.001). After adjusting for these confounders, a sustained abnormal CNFD was associated with a 3.6 V increase in VPT (95% CI, 0.3–6.9, p < 0.05) over a median duration of 4 years (1–7 years range) (Table 2). Δtriglyceride levels were excluded from the multivariable regression analysis due to collinearity. Sustained abnormal CNFL was associated with the onset of DPN (26.5% vs. 0.0%, p < 0.0001), a higher proportion of patients with burning pain, numbness, or both (67.7% vs. 34.1%, p = 0.001), and an increase in vibration perception ΔVPT (2.5 ± 10.5 vs. −0.7 ± 6.0, p = 0.05). Sustained abnormal CNFL and CNBD were non-significantly associated with a progressive loss of sudomotor function (p = 0.07) and increased neuropathic symptoms (p = 0.07).
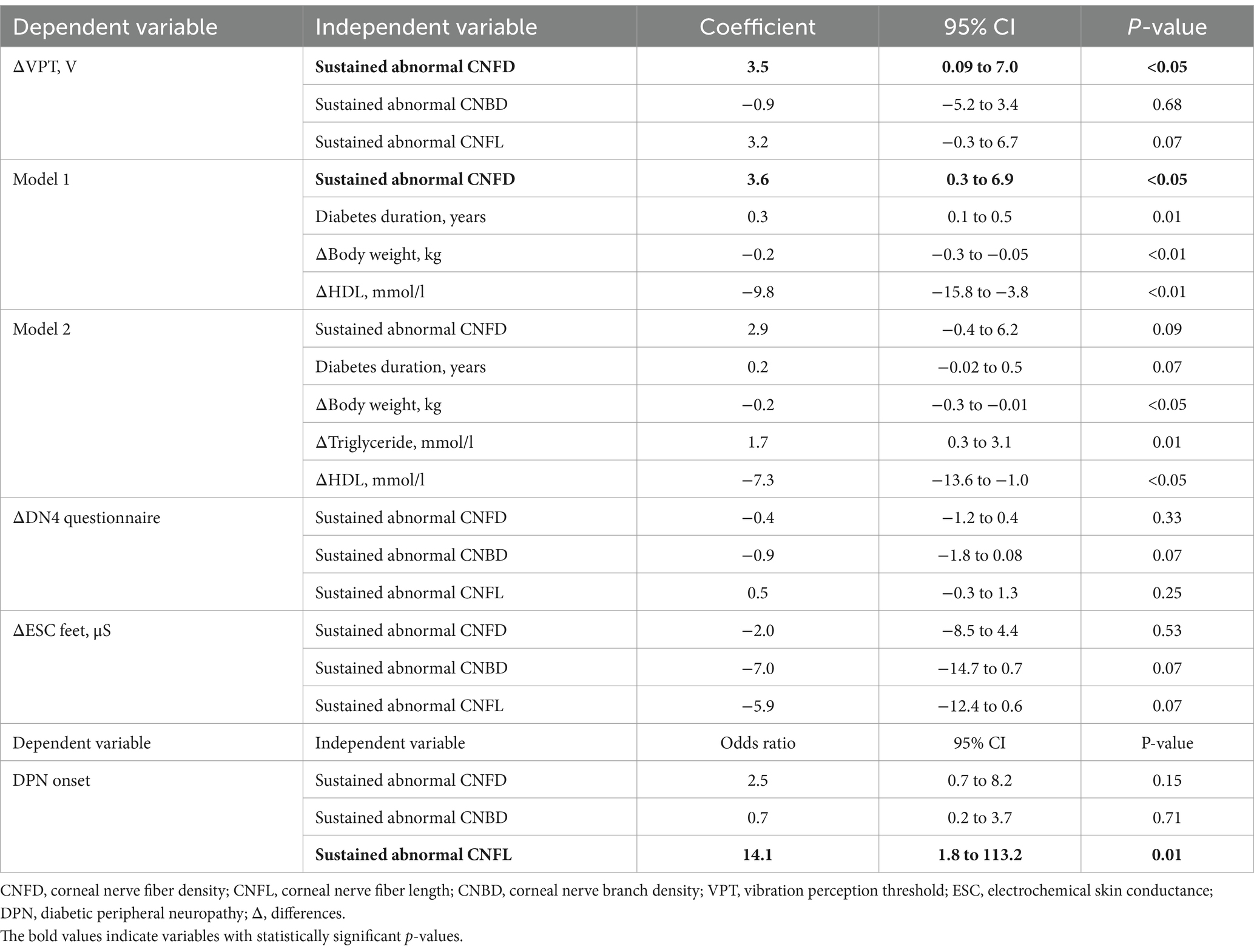
Table 2. Associations of sustained abnormal corneal nerve measures with change in neuropathy.
3.3 Predictive ability of abnormal baseline and sustained abnormal corneal nerve measures for diabetic peripheral neuropathy onsetSustained abnormal CNFL was the only measure to distinguish T2D participants who did (n = 13) and did not (n = 76) develop DPN (AUC: 76.3, 95% CI: 65.9–86.8%, p < 0.0001) (Figure 2). Abnormal baseline CNFL (AUC: 63.0, 95% CI: 46.4–79.6%, p = 0.12), abnormal baseline CNFD (AUC: 61.1, 95% CI: 44.1–78.2%, p = 0.20), abnormal baseline CNBD (AUC: 48.6, 95% CI: 31.8–65.4%, p = 0.87), sustained abnormal CNFD (AUC: 61.0, 95% CI: 44.4–77.7%, p = 0.19), and sustained abnormal CNBD (AUC: 47.8, 95% CI: 31.1–64.5%, p = 0.80) did not predict the development of DPN.
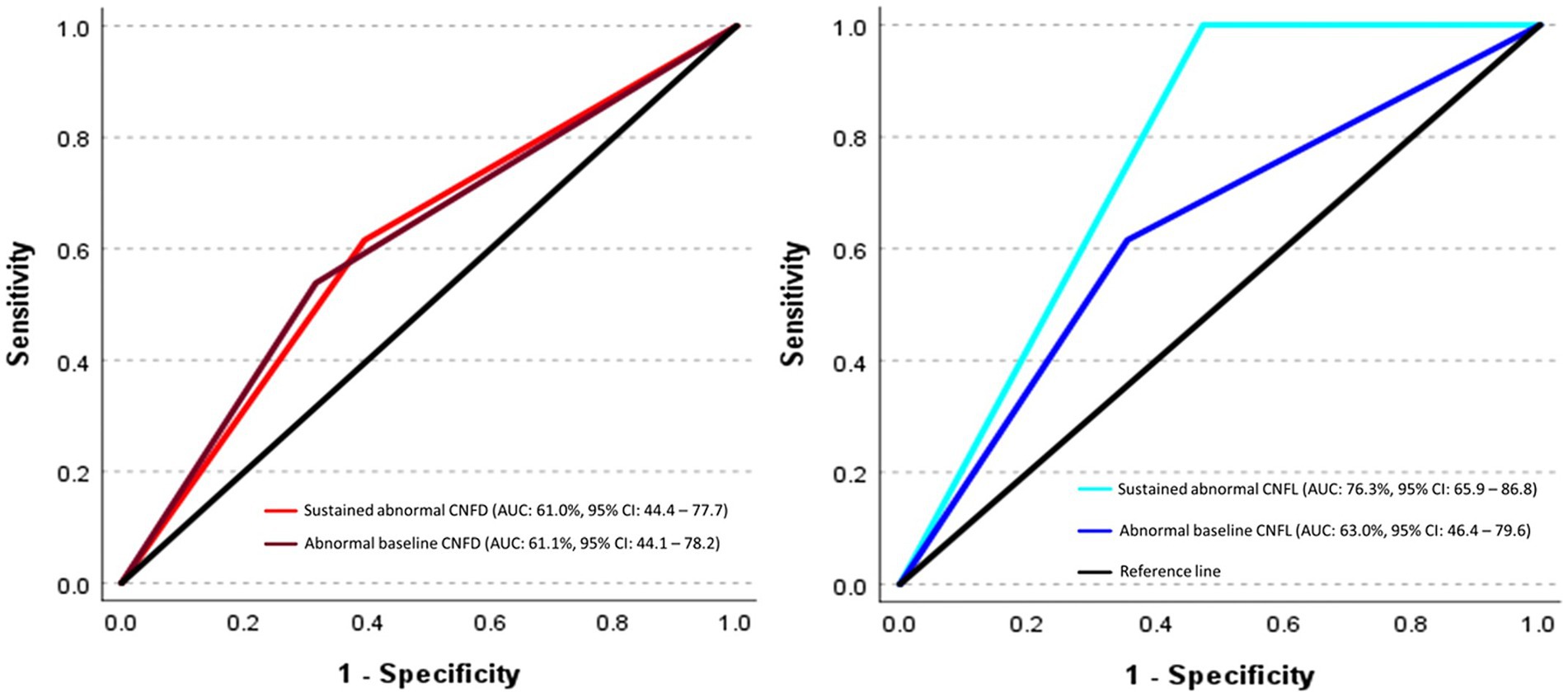
Figure 2. ROC curves show the predictive ability of abnormal baseline and sustained abnormal corneal nerve measures for incident diabetic peripheral neuropathy. The ROC analysis is expressed as the area under the curve (AUC) for corneal nerve fiber measures in discriminating between individuals who did and did not develop diabetic peripheral neuropathy (DPN).
4 DiscussionA key finding of this study is that sustained corneal nerve damage predicts the onset of DPN and the progression of neuropathic symptoms and deficits in patients with T2D. Of the three CCM measures, sustained abnormal CNFL had the highest predictive ability for DPN onset, while abnormal baseline CNFL had limited predictive capacity.
The predictive utility of CCM for incident DPN has been substantiated in two independent studies (Pritchard et al., 2015; Perkins et al., 2021). In a longitudinal diagnostic multinational consortium study (Perkins et al., 2021), the area under the ROC curve (AUC) of CNFL for incident DPN in T2D was 60–65% over 2–4 years and improved to 75% at year 6. The LANDMark study (Pritchard et al., 2015) reported an AUC of 66% (95% CI, 50–80%) for CNFL for incident DPN in type 1 diabetes (T1D) over 4 years. Both of these studies used only the baseline CNFL to predict the development of DPN. However, because more effective management of HbA1c, lipids, body weight, and physical activity can limit progressive corneal nerve damage (Ponirakis et al., 2020, 2022), this study determined whether sustained abnormal corneal nerve morphology could more reliably predict DPN development. Sustained abnormal CNFL was the only measure that predicted DPN onset with an AUC of 76.3%, while abnormal baseline CNFL had an AUC of 63%.
Corneal nerve measures at baseline were significantly lower in those with sustained corneal nerve damage, suggesting that sustained abnormalities reflect chronic irreversible neurodegeneration, while non-sustained loss of nerve fibers may reflect a capacity to regenerate, which may be relevant to the selection of patients in clinical trials of disease-modifying therapies. CNFL over 36 months of follow-up in healthy individuals remains stable (Dehghani et al., 2014), and a meta-analysis (Gad et al., 2022) showed that CNFL ranges from 16 to 21 mm/mm2 using manual analysis and from 12 to 17 mm/mm2 using automated analysis and remains stable. The average CNFL of 14.4 ± 5.2 mm/mm2 in those with sustained abnormal CNFL aligns closely with the previously determined optimal CNFL cutoff value of 14.1 mm/mm2 for incident DPN (Pritchard et al., 2015; Perkins et al., 2021), and thus, a CNFL value <14 mm/mm2 could be used to identify individuals at risk of developing DPN.
Corneal nerve damage is associated with the presence and severity of painful diabetic neuropathy (Kalteniece et al., 2022), and intraepidermal nerve fiber loss in skin biopsies has also been associated with DPN (Chen et al., 2015; Alam et al., 2017), but to a lesser extent with painful diabetic neuropathy (Thomas et al., 2023). In the current study, sustained corneal nerve damage was associated with an increased incidence of burning pain and numbness, or a combination of both, with only a moderate association with sudomotor dysfunction. There was a high prevalence of burning pain and numbness, consistent with previous studies showing a high prevalence of painful DPN in Qatar (37.5%) (Ponirakis et al., 2021) and in a multicenter study from the Gulf region (43.3%) (Ponirakis et al., 2022), attributed to poor glycemic control and obesity in the region. Corneal nerve loss is evident in pre-diabetes (Azmi et al., 2015) and recently diagnosed T2D (Pritchard et al., 2015; Perkins et al., 2021). However, corneal nerve measures show limited correlation with intraepidermal nerve fiber density (IENFD) and quantitative sensory testing (QST) (Ziegler et al., 2014; Gylfadottir et al., 2023) as corneal nerve degeneration and regeneration may follow different trajectories compared to intraepidermal nerves (Azmi et al., 2019) depending on the stage and severity of DPN. Indeed, we have previously shown corneal nerve regeneration occurs within 6 months of pancreas and kidney transplantation, while an improvement in IENFD and QST took over 12 months (Azmi et al., 2019). Gylfadottir et al. (2023) reported no correlation between corneal nerves and IENFD, QST, or the severity of neuropathy in patients with recently diagnosed and well-controlled T2D with minimal DPN, and Ziegler et al. also showed a low correlation between CCM measures and IENFD in a cohort of patients with a short duration of T2D (Ziegler et al., 2014).
This study reveals that age, elevated diastolic blood pressure, and higher HbA1c and triglycerides are predictors of sustained corneal nerve damage, which aligns with our previous multivariable regression analysis, which also showed that age, HbA1c, and body weight were predictors of reduced CNFL in T2D, while diabetes duration, LDL cholesterol, and triglycerides were predictors of reduced CNFL in T1D (Ferdousi et al., 2021). We have previously shown that physical inactivity and the use of medication-inducing weight gain are associated with a progressive decline in CNFD and CNFL in T2D (Ponirakis et al., 2022), and hypertension is independently associated with corneal nerve loss in T1D (Ponirakis et al., 2019). Thus, the evolution of corneal nerve damage and repair is highly dynamic and affected by multiple risk factors and ongoing treatment.
We acknowledge that the small sample size of the group with temporary corneal nerve damage may have limited its association with other neuropathy measures. Furthermore, the wide follow-up range of 1–7 years limits our ability to accurately determine the predictive utility of sustained abnormal CCM with incident DPN. However, the mean ± SD duration of follow-up was 3.9 ± 1.3 years, which is close to the median of 4 years, indicating a symmetric distribution of follow-up times without extreme outliers. The small SD also indicates consistency in the follow-up periods in the study. Approximately two-thirds of our cohort underwent follow-up assessments within 4–5 years, and this subset may be more reliable in exploring the association between sustained corneal nerve damage and the progression of neuropathic symptoms and deficits. The 1.5 SD cutoff for an abnormal CCM measure was chosen as the sample size for sustained abnormal CNFD (36% vs. 48%) and CNFL (39% vs. 59%) was smaller using the 2 SD compared to the 1.5 SD cutoff and would have reduced the power of the analyses. The wide range of the 95% CIs for the change in VPT with sustained abnormal CNFD and DPN onset with sustained abnormal CNFL may be attributed to abnormal lipids, high BMI, long duration of diabetes, a wide range of follow-up, and the subjective nature of the assessment of VPT. Further studies are needed to assess the association of sustained abnormal corneal nerves with IENFD, QST, and the severity of neuropathic symptoms.
In conclusion, this study shows that sustained loss of corneal nerves is associated with the development and progression of DPN, and sustained abnormal CNFL has the highest predictive ability for DPN onset. Regular monitoring of corneal nerve morphology may help to identify individuals at greater risk for DPN who should be targeted for risk factor reduction and may also help identify individuals who are more or less likely to respond to disease-modifying therapies in clinical trials.
Data availability statementCoded data from this study is available under data transfer agreement to any researcher. The corresponding author may be contacted to request access.
Ethics statementThe studies involving humans were approved by the Weill Cornell Medicine Qatar IRB and Hamad Medical Corporation IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributionsGP: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. IA-J: Writing – review & editing, Supervision, Resources, Project administration, Investigation. EE: Writing – review & editing, Investigation, Data curation. MH: Writing – review & editing, Investigation, Formal analysis, Data curation. IP: Writing – review & editing, Investigation. HG: Writing – review & editing, Investigation. AK: Visualization, Writing – review & editing, Investigation. HZ: Writing – review & editing, Investigation. HA: Writing – review & editing, Investigation. MS: Writing – review & editing, Investigation. FFSM: Writing – review & editing, Investigation. LA: Writing – review & editing, Investigation. YD: Writing – review & editing, Investigation. AS: Writing – review & editing, Investigation. RS: Writing – review & editing, Investigation. FM: Writing – review & editing, Investigation. SA-T: Writing – review & editing, Investigation. FA: Writing – review & editing, Investigation. RH: Writing – review & editing, Investigation. SM: Writing – review & editing, Investigation. NH: Writing – review & editing, Investigation. AO: Writing – review & editing, Investigation. IS: Writing – review & editing, Investigation. ZM: Writing – review & editing, Formal analysis. MZ: Writing – review & editing, Investigation. YA-A: Writing – review & editing, Investigation. SA: Writing – review & editing, Investigation, Funding acquisition. RM: Writing – review & editing, Writing – original draft, Supervision, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Qatar National Research Fund (BMRP-5726113101) and the Qatar National Research Fund (NPRP-8-315-3-065).
AcknowledgmentsThe authors thank the nurses, dieticians, and diabetes educators in the National Diabetes Center for their support and all the participants for participating in the study.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ReferencesAlam, U., Jeziorska, M., Petropoulos, I. N., Asghar, O., Fadavi, H., Ponirakis, G., et al. (2017). Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS One 12:e0180175. doi: 10.1371/journal.pone.0180175
PubMed Abstract | Crossref Full Text | Google Scholar
Azmi, S., Ferdousi, M., Petropoulos, I. N., Ponirakis, G., Alam, U., Fadavi, H., et al. (2015). Corneal confocal microscopy identifies small-Fiber neuropathy in subjects with impaired glucose tolerance who develop type 2 diabetes. Diabetes Care 38, 1502–1508. doi: 10.2337/dc14-2733
PubMed Abstract | Crossref Full Text | Google Scholar
Azmi, S., Jeziorska, M., Ferdousi, M., Petropoulos, I. N., Ponirakis, G., Marshall, A., et al. (2019). Early nerve fibre regeneration in individuals with type 1 diabetes after simultaneous pancreas and kidney transplantation. Diabetologia 62, 1478–1487. doi: 10.1007/s00125-019-4897-y
PubMed Abstract | Crossref Full Text | Google Scholar
Chen, X., Graham, J., Dabbah, M. A., Petropoulos, I. N., Ponirakis, G., Asghar, O., et al. (2015). Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care 38, 1138–1144. doi: 10.2337/dc14-2422
PubMed Abstract | Crossref Full Text | Google Scholar
Dabbah, M. A., Graham, J., Petropoulos, I. N., Tavakoli, M., and Malik, R. A. (2011). Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med. Image Anal. 15, 738–747. doi: 10.1016/j.media.2011.05.016
PubMed Abstract | Crossref Full Text | Google Scholar
Dehghani, C., Pritchard, N., Edwards, K., Vagenas, D., Russell, A. W., Malik, R. A., et al. (2014). Morphometric stability of the corneal subbasal nerve plexus in healthy individuals: a 3-year longitudinal study using corneal confocal microscopy. Invest. Ophthalmol. Vis. Sci. 55, 3195–3199. doi: 10.1167/iovs.14-13959
PubMed Abstract | Crossref Full Text | Google Scholar
Ferdousi, M., Kalteniece, A., Azmi, S., Petropoulos, I. N., Ponirakis, G., Alam, U., et al. (2021). Diagnosis of neuropathy and risk factors for corneal nerve loss in type 1 and type 2 diabetes: a corneal confocal microscopy study. Diabetes Care 44, 150–156. doi: 10.2337/dc20-1482
PubMed Abstract | Crossref Full Text | Google Scholar
Gad, H., Petropoulos, I. N., Khan, A., Ponirakis, G., MacDonald, R., Alam, U., et al. (2022). Corneal confocal microscopy for the diagnosis of diabetic peripheral neuropathy: a systematic review and meta-analysis. J. Diab. Investig. 13, 134–147. doi: 10.1111/jdi.13643
PubMed Abstract | Crossref Full Text | Google Scholar
Guldiken, Y. C., Malik, A., Petropoulos, I. N., Gad, H., Elgassim, E., Salivon, I., et al. (2023). Where art thou O treatment for diabetic neuropathy: the sequel. Expert. Rev. Neurother. 23, 845–851. doi: 10.1080/14737175.2023.2247163
PubMed Abstract | Crossref Full Text | Google Scholar
Gylfadottir, S. S., Itani, M., Kristensen, A. G., Nyengaard, J. R., Sindrup, S. H., Jensen, T. S., et al. (2023). Assessing corneal confocal microscopy and other small Fiber measures in diabetic polyneuropathy. Neurology 100, e1680–e1690. doi: 10.1212/WNL.0000000000206902
PubMed Abstract | Crossref Full Text | Google Scholar
Kalteniece, A., Ferdousi, M., Adam, S., Schofield, J., Azmi, S., Petropoulos, I., et al. (2017). Corneal confocal microscopy is a rapid reproducible ophthalmic technique for quantifying corneal nerve abnormalities. PLoS One 12:e0183040. doi: 10.1371/journal.pone.0183040
PubMed Abstract | Crossref Full Text | Google Scholar
Kalteniece, A., Ferdousi, M., Azmi, S., Khan, S. U., Worthington, A., Marshall, A., et al. (2022). Corneal nerve loss is related to the severity of painful diabetic neuropathy. Eur. J. Neurol. 29, 286–294. doi: 10.1111/ene.15129
PubMed Abstract | Crossref Full Text | Google Scholar
Lewis, E. J. H., Lovblom, L. E., Ferdousi, M., Halpern, E. M., Jeziorska, M., Pacaud, D., et al. (2020). Rapid corneal nerve fiber loss: a marker of diabetic neuropathy onset and progression. Diabetes Care 43, 1829–1835. doi: 10.2337/dc19-0951
PubMed Abstract | Crossref Full Text | Google Scholar
Mallik, A., and Weir, A. I. (2005). Nerve conduction studies: essentials and pitfalls in practice. J. Neurol. Neurosurg. Psychiatry 76, ii23–ii31. doi: 10.1136/jnnp.2005.069138
Crossref Full Text | Google Scholar
Perkins, B. A., Lovblom, L. E., Lewis, E. J. H., Bril, V., Ferdousi, M., Orszag, A., et al. (2021). Corneal confocal microscopy predicts the development of diabetic neuropathy: a longitudinal diagnostic multinational consortium study. Diabetes Care 44, 2107–2114. doi: 10.2337/dc21-0476
Crossref Full Text | Google Scholar
Petropoulos, I. N., Manzoor, T., Morgan, P., Fadavi, H., Asghar, O., Alam, U., et al. (2013). Repeatability of in vivo corneal confocal microscopy to quantify corneal nerve morphology. Cornea 32, e83–e89. doi: 10.1097/ICO.0b013e3182749419
PubMed Abstract | Crossref Full Text | Google Scholar
Ponirakis, G., Abdul-Ghani, M. A., Jayyousi, A., Almuhannadi, H., Petropoulos, I. N., Khan, A., et al. (2020). Effect of treatment with exenatide and pioglitazone or basal-bolus insulin on diabetic neuropathy: a substudy of the Qatar study. BMJ Open Diabetes Res. Care 8:e001420. doi: 10.1136/bmjdrc-2020-001420
PubMed Abstract | Crossref Full Text | Google Scholar
Ponirakis, G., Al-Janahi, I., Elgassim, E., Gad, H., Petropoulos, I. N., Khan, A., et al. (2022). Progressive loss of corneal nerve fibers is associated with physical inactivity and glucose lowering medication associated with weight gain in type 2 diabetes. J. Diab. Investig. 13, 1703–1710. doi: 10.1111/jdi.13864
PubMed Abstract | Crossref Full Text | Google Scholar
Ponirakis, G., Elhadd, T., Al Ozairi, E., Brema, I., Chinnaiyan, S., Taghadom, E., et al. (2022). Prevalence and risk factors for diabetic peripheral neuropathy, neuropathic pain and foot ulceration in the Arabian gulf region. J. Diab. Investig. 13, 1551–1559. doi: 10.1111/jdi.13815
PubMed Abstract | Crossref Full Text | Google Scholar
Ponirakis, G., Elhadd, T., Chinnaiyan, S., Hamza, A. H., Sheik, S., Kalathingal, M. A., et al. (2021). Prevalence and risk factors for diabetic neuropathy and painful diabetic neuropathy in primary and secondary healthcare in Qatar. J. Diab. Investig. 12, 592–600. doi: 10.1111/jdi.13388
PubMed Abstract | Crossref Full Text | Google Scholar
Ponirakis, G., Petropoulos, I. N., Alam, U., Ferdousi, M., Asghar, O., Marshall, A., et al. (2019). Hypertension contributes to neuropathy in patients with type 1 diabetes. Am. J. Hypertens. 32, 796–803. doi: 10.1093/ajh/hpz058
PubMed Abstract | Crossref Full Text | Google Scholar
Pop-Busui, R., Boulton, A. J., Feldman, E. L., Bril, V., Freeman, R., Malik, R. A., et al. (2017). Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 40, 136–154. doi: 10.2337/dc16-2042
PubMed Abstract | Crossref Full Text | Google Scholar
Pritchard, N., Edwards, K., Russell, A. W., Perkins, B. A., Malik, R. A., and Efron, N. (2015). Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care 38, 671–675. doi: 10.2337/dc14-2114
PubMed Abstract | Crossref Full Text | Google Scholar
Selvarajah, D., Cash, T., Davies, J., Sankar, A., Rao, G., Grieg, M., et al. (2015). SUDOSCAN: a simple, rapid, and objective method with potential for screening for diabetic peripheral neuropathy. PLoS One 10:e0138224. doi: 10.1371/journal.pone.0138224
PubMed Abstract | Crossref Full Text | Google Scholar
Spallone, V., Morganti, R., D'Amato, C., Greco, C., Cacciotti, L., and Marfia, G. A. (2012). Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet. Med. 29, 578–585. doi: 10.1111/j.1464-5491.2011.03500.x
PubMed Abstract | Crossref Full Text | Google Scholar
Thomas, S. J., Enders, J., Kaiser, A., Rovenstine, L., Heslop, L., Hauser, W., et al. (2023). Abnormal intraepidermal nerve fiber density in disease: a scoping review. medRxiv. doi: 10.3389/fneur.2023.1161077
Crossref Full Text | Google Scholar
Werner, M. U., Petersen, M. A., and Bischoff, J. M. (2013). Test-retest studies in quantitative sensory testing: a critical review. Acta Anaesthesiol. Scand. 57, 957–963. doi: 10.1111/aas.12150
PubMed Abstract | Crossref Full Text | Google Scholar
Ziegler, D., Papanas, N., Zhivov, A., Allgeier, S., Winter, K., Ziegler, I., et al. (2014). Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes 63, 2454–2463. doi: 10.2337/db13-1819
Comments (0)