1Department of Pharmacology, Swamy Vivekanandha College of
2Pharmacy, Tiruchengode, Namakkal-637 205, Tamil Nadu, India
Corresponding author email: sudhakar00pharma@gmail.com
Article Publishing HistoryReceived: 15/11/2023
Accepted After Revision: 29/03/2024
ABSTRACT:Gymnema sylvestre (Apocynaceae) is a well-known anti-diabetic herb used in various traditional Indian medicinal systems including Ayurveda and modern medicine. It is a source of diverse phytoconstituents and was reported to be used to treat various diseases. Hence our study aimed to evaluate the anti-inflammatory and antioxidant properties of Gymnema sylvestre by in vitro techniques. The ethanolic leaves extract of G. sylvestre was preliminarily screened to identify the presence of various phytoconstituents, and further analyzed for total alkaloids, flavonoids, phytosterols, and saponins estimations. The in vitro anti-inflammatory activity was assayed by human red blood cell (HRBC) membrane stabilization and protein denaturation assay, while antioxidant activity was assessed by DPPH and nitric oxide scavenging assay.
Our study results showed that the ethanolic leaf extract of G. sylvestre exhibited dose-dependent anti-inflammatory and antioxidant activity. Preliminary phytochemical screening revealed the presence of alkaloids, flavonoids, phytosterols, tannins, phenolic compounds, terpenoids, glycosides, carbohydrates, sugars, proteins, amino acids, and saponins. Quantitative analysis showed high content of saponin 32.44 ± 5.65 mg diosgenin/g, followed by steroidal content 26.30 ± 3.96 mg cholesterol/g, flavonoids 17.66 ± 0.43 mg QE/g, and alkaloids 10.33 ± 0.97 mg AE/g. These results further authenticate the claim and use of G. sylvestre in traditional medicine to treat inflammation and oxidative stress.
KEYWORDS:Antioxidant, Anti-Inflammatory, Gymnema Sylvestre, Dpph, Nitric Oxide.
Download this article as: Copy the following to cite this article:Pachiappan S, Kodali S, Palanisamy S, Chandrasekar S. In Vitro Evaluations of Anti-inflammatory and Antioxidant Activity of Ethanolic Leaf Extract of Gymnema sylvestre R. Br. Biosc.Biotech.Res.Comm. 2024;17(1).
Copy the following to cite this URL:Pachiappan S, Kodali S, Palanisamy S, Chandrasekar S. In vitro Evaluations of Anti-inflammatory and Antioxidant Activity of Ethanolic Leaf Extract of Gymnema sylvestre R. Br. Biosc.Biotech.Res.Comm. 2023;17(1). Available from: <a href=”https://shorturl.at/nqrNV“>https://shorturl.at/nqrNV</a>
INTRODUCTION
Oxidative stress has a significant role in chronic inflammatory illnesses such as cancer, diabetes, neurological problems, and cardiovascular ailments. Prolonged exposure to high quantities of pro-oxidants can cause mitochondrial DNA damage and changes in cellular components, which contribute to gene expression anomalies (Sharifi-Rad et al., 2020). The process of oxidative stress that leads to the damage of numerous tissue’s physiological and biochemical environments, a minor amount of oxidative stress that helps the immune system withstand microbial infections and intracellular cell signalling, is also thought to be an important physiological activity (Sies et al., 2017). When free radicals outnumber antioxidant defences, oxidative stress, a process that harms the physiological and biochemical balance of tissues, becomes worse (Kopáni et al., 2006). Inflammation increases reactive oxygen species (ROS) generation, which exceeds the body’s antioxidant capacity and causes oxidative stress-induced tissue damage (Sies 1997).
Early in the ages, medicinal plants have been extensively used to treat a wide range of diseases, notably in India, where indigenous medical systems such as Ayurveda, Siddha, and Unani have been practiced for centuries. Herbalism, or the medicinal use of herbal plants, is important in modern medicine. While synthetic drugs provide treatments for a variety of illnesses, their large quantity and related side effects sometimes limit accessibility. The use of herbs, on the other hand, is gaining popularity because of low toxicity, low cost, and broad availability, emphasizing the importance of herbs in everyday life (Das et al., 2022).
Nearly 80% of the world’s population gets their basic medical treatment from medicinal plants, according to the World Health Organisation (WHO), highlighting the long-standing importance of herbal medicine in treating illnesses affecting human health (Dey et al., 2021). Traditional methods of treating inflammatory illnesses mainly target the fight against bacterial infections, which might not be effective enough. As a result, complementary approaches that target both inflammation and oxidative stress reduction have developed as viable therapeutic and preventative treatments.
In this context, antioxidant compounds, which may be found in a variety of foods, drinks, plants, vitamins, and minerals are essential. Both conventional insight and contemporary studies have highlighted the anti-inflammatory and antioxidant qualities of medicinal plants. Despite their effectiveness, modern anti-inflammatory drugs can have a variety of negative effects. On the other hand, because of their natural source, herbal extracts are thought to provide a safer substitute. Treating chronic inflammatory diseases is made easier when a single plant extract combines anti-inflammatory and antioxidant effects (Somashekar et al., 2022).
Gymnema sylvestre (G. sylvestre), Apocynaceae family, is a well-established antidiabetic herb utilized in various traditional Indian medicinal systems including Ayurveda and modern medicine. Known as “Gudmar,” this herbaceous plant is a climbing species found in dry forests up to 600 meters in height. Its leaves are extensively employed for their multifaceted therapeutic properties, encompassing antidiabetic, anti-inflammatory, antiarthritic, anti-obesity, wound healing, astringent, bitter, acrid, thermogenic, anodyne, digestive, antipyretic, stomachic, diuretic, laxative, cardiotonic, and liver tonic effects. Rich in tannins, flavonoids, saponins, and gymnemic acid, G. sylvestre exhibits additional bioactive characteristics such as antimicrobial, larvicidal, antiviral, hypolipidemic, anticancer, and antioxidant activities. particularly gymnemic acids, a blend of at least 17 distinct saponins, acidic glycosides, and anthraquinones (Sudhakar et al., 2018, Pachiappan et al., 2021 Pachiappan et al., 2023).
The research regarding the pharmacological effects of G. sylvestre is currently at an early stage and requires further investigation to fully understand the phytochemical advantages of this herb. This in vitro study is designed to examine the antioxidative and anti-inflammatory properties of G. sylvestre leaf ethanolic extract.
MATERIALS AND METHODS
Plant material: The fresh leaves of Gymnema sylvestre R. Br. (GS) were collected from Kolli Hills Namakkal, Tamil Nadu, India, during January 2021. The plant material was further authenticated by Dr. P. Radha, Research officer (Botany), Siddha Medicinal Plants Garden (Central Council for Research in Siddha), Ministry of AYUSH, Govt. of India, Mettur Dam, Tamil Nadu, India, where the voucher specimen is preserved with the reference number of G180221012S.
Preparation of extract: The collected fresh leaves were washed with running water to remove the sand and dust. Then the shade-dried material was coarsely powdered for extraction. 100 gm of the powdered plant material was loaded in the Erlenmeyer flask and extracted by cold maceration with 95% ethanol for 72 hours at room temperature with occasional shaking. After 72 hours, the filtrate was separated from marc by using a muslin cloth and further filtered by Whatman no. 1 filter paper. The same procedure was performed two consecutive times with the marc material. All three filtrates were mixed and evaporated under reduced pressure and controlled temperature at 40 0C in a rotary evaporator until all the solvent was removed. The dried material was stored in an airtight container at 4 0C until further use.
Preliminary phytochemical analysis of ethanolic extract of Gymnema sylvestre: The preliminary phytochemical screening of G. sylvestre leaves ethanolic extract was carried out using standard procedure to identify the presence of alkaloids, flavonoids, phytosterols, tannins, phenolic compounds, terpenoids, glycosides, carbohydrates, sugars, proteins, amino acids, and saponins (Evans & WC 2009, Kokate et al., 2017).
Quantitative phytochemical analysis: Determination of total alkaloid content: The extract (1 mg/ml), bromocresol green solution, and phosphate buffer 5 ml each were mixed in a separating funnel. It was diluted with chloroform in 10 ml volumetric flask. Atropine was used as standard. The absorbance of both the standard and test solution was observed at 470 nm. The total alkaloid content in the extract was expressed as mg of Atropine (AE)/g of plant extract (Shalini et al., 2021).
Determination of total flavonoid content: The plant extract 3 mg was dissolved in methanol 3 ml for flavonoid content estimation. The quercetin was used as standard. 3 ml of plant extract or standard was mixed with 1 ml of 2% AlCl3 methanolic solution and allowed to stand for 60 min at room temperature. The absorbance was measured at 420 nm. The total flavonoid content was indicated as mg of QE/g of extract (Garg & Garg 2019).
Determination of total steroidal content: The total steroidal content was estimated by Liebermann-burchard colorimetric method with slight modification. The G. sylvestre extract was dissolved with chloroform and the freshly prepared Liebermann-burchard reagent was added. The absorbance of standard cholesterol and extract was measured at 650 nm against a reagent blank. The total steroidal content was expressed as mg of cholesterol/g of extract (Kim & Goldberg 1969).
Determination of total saponin content: The 1 ml of diluted G. sylvestre extract was mixed with 1 ml of 80% aqueous methanol, followed by 1 ml of 72% H2So4 added in the sides of test tube. The mixture was warmed on 60 0C for 10 minutes. The absorbance of standard diosgenin and extract solution was measured at 544 nm against 80% methanol as a blank solution. The total saponin content was expressed as mg of diosgenin equivalent to gm of dry weight of the extract (Nandhini & Ilango 2020).
In vitro anti-inflammatory activity screening : Human red blood cell (HRBC) membrane stabilization assay: The in vitro anti-inflammatory activity of G. sylvestre was determined by the Human Red Blood Cell (HRBC) membrane stabilization method. The blood sample was collected from a healthy human volunteer who had not taken any NSAIDs for 2 weeks before the experiment, added with an equal amount of Alsever’s solution. This mixture was centrifuged at 3000 rpm for 15 min. The RBC pellet was washed thrice with sterile saline till the supernatant was clear and colorless.
The packed cellular content was formulated as 10 % v/v suspension with sterile isosaline. 1 ml of different concentrations (100-500 µg/ml) of G. sylvestre extract and standard diclofenac was mixed with 1 ml of phosphate buffer, 0.5 ml of HRBC suspension, and 2 ml of hyposaline. After 30 min incubation at 37 0C, the reaction mixture was centrifuged at 3000 rpm for 10 min. The supernatant absorbance was observed spectrophotometrically at 560 nm. The percentage of hemolysis was estimated by considering the percentage of hemolysis of control as 100% (Senthil Kumar et al., 2018; Gupta et al., 2021). The percentage of protection/ percentage inhibition of hemolysis were evaluated using the formula.
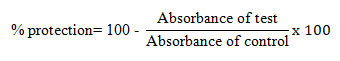
Protein denaturation assay: The in vitro anti-inflammatory activity of the G. sylvestre extracts by protein denaturation was performed using bovine serum albumin. The increasing concentrations of the extract (100-500 µg/ml) and reference compound diclofenac sodium were incubated with 0.5% w/v of bovine serum albumin at 37◦C for 20 min and the temperature was increased to keep the samples at 57 ◦C for 30 min. After reaching room temperature, the turbidity was measured using UV-Visible spectrophotometer at 660 nm following the addition of 2.5 ml of phosphate-buffered saline (Shanmuganathan et al., 2017; Senthil Kumar et al., 2018). The percentage inhibition of protein denaturation was calculated by using the following formula.
![]()
In vitro antioxidant activity screening :DPPH free radical scavenging assay: The 2, 2 diphenyl-1-picrylhydrazyl (DPPH) 0.2mM solution 1 ml was added to 1 ml of different concentrations (100-500 µg/ml) of G. sylvestre extract, the mixture was kept room temperature for 50 mins in dark environment. The antioxidant activity was measured spectrophotometrically at 517 nm. Ascorbic acid was used as standard. The percentage of free radical scavenging was calculated as half minimal inhibitory concentration (IC50). IC50 denotes the concentration of the sample required to inhibit 50% of DPPH free radicals (Govindappa et al., 2018). The DPPH radical scavenging capacity was calculated by using the following formula:
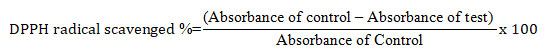
Nitric oxide (NO) radical scavenging activity: The nitric oxide scavenging assay reaction mixture (3 ml) contains 2 ml of 10 mM sodium nitroprusside, 0.5 ml of phosphate buffer, and 0.5 ml of different concentrations (100-500 µg/ml) of G. sylvestre extract. The reaction mixture was incubated at 25 0C for 150 min. The 0.5 ml of reaction mixture was pipetted and mixed with 1 ml of sulfanilic acid reagent and allowed to stand for 5 min for diazotization. Then 1 ml of naphthyl ethylene diamine dihydrochloride was added and incubated at 25 0C for 30 min. A pink-colored chromophore was formed in diffused light. Ascorbic acid was used as standard. The NO scavenging activity was measured at 550 nm and the results were expressed as a percentage (%) of scavenging using the following formula: (Adebayo et al., 2019)
![]()
Statistical analysis: The statistical analysis was performed in IBM SPSS version 18 (SPSS version 18.0; IBM Corporation, Armonk, NY, USA). The entire assay was performed in triplicate and the values were expressed as mean ± standard deviation (SD). Descriptive statistics were used for continuous variables and expressed in mean and standard deviation. A test of normality was applied.
RESULTS AND DISCUSSION
Extractive yield and preliminary phytochemical analysis: The ethanolic extractive percentage yield of Gymnema sylvestre leaf extract was calculated as 22.08 gm% w/w. The preliminary phytochemical analysis of ethanolic leaves extract of G. sylvestre showed the presence of alkaloids, flavonoids, phytosterols, tannins, phenolic compounds, terpenoids, glycosides, carbohydrates, sugars, proteins, amino acids, and saponins.
Quantitative phytochemical analysis: Table 1 illustrates the quantification of secondary metabolites alkaloids, flavonoids, steroids, and saponins in the ethanolic leaves extract of G. sylvestre. The secondary metabolite saponins content was found to be higher 32.44 ± 5.65 mg diosgenin/g, followed by steroidal content 26.30 ± 3.96 mg cholesterol/g, flavonoids 17.66 ± 0.43 mg QE/g, and alkaloids 10.33 ± 0.97 mg AE/g in the ethanolic leaves extract of G. sylvestre.
Table 1. Quantitative phytochemical analysis of G. sylvestre
leaves ethanolic extract
(mg/g of extract)
1. Alkaloids 10.33 ± 0.97 2. Flavonoids 17.66 ± 0.43 3. Steroids 26.30 ± 3.96 4. Saponins 32.44 ± 5.65Values are expressed as mean ± SD, n=3.
In vitro anti-inflammatory activity: The in vitro anti-inflammatory activity of G. sylvestre was carried out by Human red blood cell membrane stabilization assay and protein denaturation assay techniques.
HRBC membrane stabilization assay: HRBC assay is based on the principle of evaluating lysosomal membrane protection. During inflammation lysosomes undergo lysis and release specific enzymes into circulation leading to inflammatory diseases. The RBC membrane is similar to the lysosomal membrane, and its stability indicates anti-inflammatory properties (Yesmin et al., 2020). In this study, the hemolysis of RBC was impacted by the hypotonicity of hyposaline, which induces lysis of the cell membrane. The percentage of RBC lysis/protection was taken as an index for anti-inflammatory activity measurement (Kumar et al., 2011 and Kumar et al., 2020).
The treatment of G. sylvestre showed dose-dependent membrane stabilization action the maximum activity (72.46 ± 2.58) was observed at the concentration 500 µg/ml. The standard diclofenac showed maximum membrane protection (89.27 ± 1.08) activity at the concentration 500 µg/ml, comparably the treatment of G. sylvestre leaves extract showed less percentage inhibition of hemolysis compared to the standard diclofenac. The treatment of G. sylvestre ethanolic leaves extract exhibited remarkable anti-inflammatory activity by stabilizing the RBC membrane, preventing the discharge of lytic enzymes and other inflammation mediators (Table 2).
Table 2. Human red blood cell membrane stabilization assay of
G. sylvestre leaves ethanolic extract
(µg/ml)
% Protection Ethanolic extract of GS Diclofenac(Standard)
1. 100 23.51 ± 1.01 48.11 ± 2.77 2. 200 34.25 ± 0.98 66.96 ± 2.54 3. 300 40.19 ± 1.50 75.39 ± 7.80 4. 400 56.88 ± 3.55 78.93 ± 4.13 5. 500 72.46 ± 2.58 89.27 ± 1.08Values are expressed as mean ± SD, n=3.
Protein denaturation assay: The denaturation of cellular protein is the most known cause of inflammation and arthritis. The compounds that prevent the protein denaturation have advantageous anti-inflammatory properties (Osman et al., 2016). The results in table 3 shows that maximum (71.89 ± 7.89) percentage denaturation inhibition was observed in the G. sylvestre 500 µg/ml treatment, when compared to standard diclofenac it shows maximum inhibition (78.70 ± 2.81) at the concentration of 500 µg/ml which is almost equal. The ethanolic leaves extract of G. sylvestre showed dose dependent protein denaturation inhibition property. As results the protein denaturation inhibition capacity confirms the anti-inflammatory property of G. sylvestre extract.
Table 3. Protein denaturation inhibition assay of G. sylvestre
leaves ethanolic extract
(µg/ml)
% inhibition Ethanolic extract of GS Diclofenac(Standard)
1. 100 18.88 ± 1.42 24.71 ± 3.31 2. 200 27.12 ± 1.32 39.14 ± 6.04 3. 300 38.41 ± 0.70 52.90 ± 1.11 4. 400 58.62 ± 2.45 67.22 ± 0.83 5. 500 71.89 ± 7.89 78.70 ± 2.81Values are expressed as mean ± SD, n=3.
In vitro antioxidant assay
The antioxidant potential of G. sylvestre leaves extract was assayed by DPPH free radical scavenging assay and Nitric oxide (NO) radical scavenging assay.
DPPH free radical scavenging assay: DPPH radical scavenging is the most widely used technique for measuring free radical scavenging. The antioxidant ability of compounds was determined by their capacity to donate hydrogen was assumed to be responsible for DPPH scavenging. The DPPH antioxidant model may have some positive benefits in certain inflammatory disorders (Nagulsamy et al., 2015, Kawra et al., 2020). The G. sylvestre leaf extract and ascorbic acid showed a dose-response relationship with the DPPH scavenging activity which was directly proportional to their concentrations. The IC50 value for ascorbic acid was 196.88 µg/ml, which was significantly lower than the G. sylvestre extract 289.15 µg/ml (Table 4).
Table 4. DPPH radical scavenging activity of G. sylvestre
leaves ethanolic extract
(µg/ml)
% scavenging activity Ethanolic extract of GS Ascorbic acid(Standard)
1. 100 27.32 ± 2.56 32.07 ± 1.22 2. 200 38.58 ± 5.92 59.33 ± 5.05 3. 300 46.65 ± 9.02 62.43 ± 3.25 4. 400 68.45 ± 4.52 74.21 ± 3.82 5. 500 77.32 ± 6.29 82.91 ± 6.64 IC50 289.15 ± 11.45 196.38 ± 8.19Values are expressed as mean ± SD, n=3.
Nitric oxide radical scavenging assay: Inflammatory diseases have a high production of nitric oxide. Overproduction of NO can cause tissue damage and contribute to inflammatory diseases including atherosclerosis and hypertension. The compounds that can scavenge or inhibit the production of NO are known to have antioxidant properties (Moncada et al., 1991, Adebayo et al., 2019). The G. sylvestre leaf extract and standard ascorbic acid showed a dose-response relationship with the NO scavenging activity which was directly proportional to their concentrations. The IC50 value for ascorbic acid was 107.07µg/ml, which was significantly lower than the G. sylvestre extract at 258.93 µg/ml (Table 5).
Table 5. Nitric oxide radical scavenging activity of G. sylvestre
leaves ethanolic extract
(µg/ml)
% scavenging activity Ethanolic extract of GS Ascorbic acid(Standard)
1. 100 29.45 ± 0.89 48.33 ± 6.02 2. 200 40.99 ± 1.34 61.73 ± 4.05 3. 300 56.05 ± 2.62 75.00 ± 2.39 4. 400 71.30 ± 3.27 86.19 ± 4.31 5. 500 80.12 ± 6.80 97.68 ± 3.24 IC50 258.93 ± 9.06 107.07 ± 12.46Values are expressed as mean ± SD, n=3.
CONCLUSION
The results of this study demonstrated that the ethanolic leaf extract of G. sylvestre inhibits hemolysis by protecting the RBC cell membrane in HRBC membrane stabilization assay and preventing protein denaturation in protein denaturation assay. Free radical scavenging property was demonstrated through DPPH radical scavenging and nitric oxide radical scavenging in a concentration-dependent manner. These suggest that G. sylvestre leaf ethanolic extract has extensive anti-inflammatory and antioxidant potency. The presence of secondary metabolites might contribute to its actions, the free radical scavenging activity of G. sylvestre leaf extract can play a vital role in modulating inflammatory reactions. Hence Gymnema sylvestre leaf extract may utilized for the herbal medicine development to the inflammation condition associated with oxidative stress. Further, in vivo studies and the specific bioactive compound isolation are needed to explore Gymnema sylvestre as a newer therapeutic agent.
ACKNOWLEDGEMENTS
The authors are thankful to the Principal of Swamy Vivekanandha College of Pharmacy, Tamil Nadu, India for providing the necessary facilities
Consent for Publication: Not applicable.
Availability of Data and Material: All the data generated and analyzed during the study are included in the main manuscript.
Competing Interests: The authors declare that they have no competing interests.
Funding: NA
REFERENCES
Adebayo, S.A., Ondua, M., Shai, L.J. et al., (2019) Inhibition of nitric oxide production and free radical scavenging activities of four South African medicinal plants. Journal of inflammation research, pp.195-203.
Das, K., Asdaq, S.M.B., Khan, M.S., et al., (2022) Phytochemical investigation and evaluation of in vitro anti-inflammatory activity of Euphorbia hirta ethanol leaf and root extracts: A comparative study. Journal of King Saud University-Science, 34(7), p.102261.
Dey, A., Nandy, S., Mukherjee, A. et al., (2021) Sustainable utilization of medicinal plants and conservation strategies practiced by the aboriginals of Purulia district, India: a case study on therapeutics used against some tropical otorhinolaryngologic and ophthalmic disorders. Environment, Development and Sustainability, 23, pp.5576-5613.
Evans, W.C., (2009) Trease and Evans’ pharmacognosy. Elsevier Health Sciences, pp. 553-557.
Garg, P. and Garg, R., (2019) Phytochemical screening and quantitative estimation of total flavonoids of Ocimum sanctum in different solvent extract. Pharma Innov J, 8(2), pp.16-21.
Govindappa, M., Hemashekhar, B., Arthikala, M.K., et al., (2018) Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results in Physics, 9, pp.400-408.
Gupta, A., Kumar, R., Ganguly, R., et al., (2021) Antioxidant, anti-inflammatory and hepatoprotective activities of Terminalia bellirica and its bioactive component ellagic acid against diclofenac induced oxidative stress and hepatotoxicity. Toxicology reports, 8, pp.44-52.
Kawra, M., Saklani, S. and Parcha, V., (2020) Preliminary phytochemical screening and antioxidant activity of five medicinal plants of Garhwal Himalaya: a comparative study. Vegetos, 33(3), pp.610-613.
Kim, E. and Goldberg, M., (1969) Serum cholesterol assay using a stable Liebermann-Burchard reagent. Clinical chemistry, 15(12), pp.1171-1179.
Kokate C.K., Purohit A.P. and Gokhale S.B (2017) Pharmacognosy. 54th Edn. NiraliPrakashan, Pune, India. p.7.16-7.19.
Kopáni, M., Celec, P., Danišovič, L., et al., (2006) Oxidative stress and electron spin resonance. Clinica chimica acta, 364(1-2), pp.61-66.
Kumar, S.V., Kumar, R.S., Sudhakar, P. et al., (2020) Antioxidant, Antinociceptive and Anti-inflammatory activities of Rhynchosia minima (L) DC. Research Journal of Pharmacy and Technology, 13(4), pp.1855-1860.
Kumar, V., Bhat, Z.A., Kumar, D., et al., (2011) In-vitro anti-inflammatory activity of leaf extracts of Basella alba linn. Var. alba. Int J Drug Dev Res, 3(2), pp.176-179.
Moncada, S.R.M.J., Palmer, R.M.L. and Higgs, E., (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacological reviews, 43(2), pp.109-142.
Nagulsamy, P., Ponnusamy, R. and Thangaraj, P., (2015) Evaluation of antioxidant, anti-inflammatory, and antiulcer properties of Vaccinium leschenaultii Wight: A therapeutic supplement. Journal of food and drug analysis, 23(3), pp.376-386.
Nandhini, S. and Ilango, K., (2020) Comparative study on pharmacognostical, phytochemical investigations and quantification of vasicine content in the extracts of Adhatoda vasica Nees and Adhatoda beddomei CB Clarke. Pharmacognosy Journal, 12(4).
Osman, N.I., Sidik, N.J., Awal, A., et al., (2016) In vitro xanthine oxidase and albumin denaturation inhibition assay of Barringtonia racemosa L. and total phenolic content analysis for potential anti-inflammatory use in gouty arthritis. Journal of Intercultural Ethnopharmacology, 5(4), p.343.
Pachiappan, S., Ramalingam, K. and Balasubramanian, A., (2021) Combined effects of Gymnema sylvestre and Pergularia daemia on letrozole-induced polycystic ovarian syndrome in rats. Asian Pacific Journal of Reproduction, 10(2).
Pachiappan, S., Ramalingam, K. and Balasubramanian, A., (2023) Evaluation of Gymnema sylvestre R. Br. against Letrozole Induced Polycystic Ovarian Syndrome in rats. Research Journal of Pharmacy and Technology, 16(1), pp.385-390.
Senthil Kumar, R., Vinoth Kumar, S., Abdul Lathiff, M.K.M., et al., (2018) Antioxidant and anti-inflammatory activities of leaf extracts of Flacourtia jangomas (Lour.) Raeusch: An study in vitro. Advance Pharmaceutical Journal, 3(6), pp.169-176.
Shalini, K. and Ilango, K.J.P.J., (2021) Preliminary phytochemical studies, GC-MS analysis and in vitro antioxidant activity of selected medicinal plants and its polyherbal formulation. Pharmacognosy Journal, 13(3).
Shanmuganathan, T., Parthasarathy, K., Venugopal, M., et al., (2017) Synthesis, in vitro anti-inflammatory activity and molecular docking studies of novel 4, 5-diarylthiophene-2-carboxamide derivatives. Journal of Chemical Sciences, 129, pp.117-130.
Sharifi-Rad, M., Anil Kumar, N.V., Zucca, P., et al., (2020) Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Frontiers in Physiology, 11, p.694.
Sies, H., (1997) Oxidative stress: oxidants and antioxidants. Experimental Physiology: Translation and Integration, 82(2), pp.291-295.
Sies, H., Berndt, C. and Jones, D.P., (2017) Oxidative stress. annu. rev. 715-748.
Somashekar, G., Sudhakar, U., Prakash, S.G., et al., (2022) In-vitro Antioxidant and In-vitro Anti-inflammatory activities of Ethanolic leaves extract of Ormocarpum Cochinchinense. Journal of Orofacial Sciences, 14(2), pp.134-140.
Sudhakar, P., Suganeswari, M., Pushkalai, P.S. et al., (2018). Regulation of Estrous cycle using Combination of Gymnema sylvestre and Pergularia daemia in Estradiol Valerate induced PCOS rats. Asian Journal of Research in Pharmaceutical Science, 8(1), pp.4-8.
Yesmin, S., Paul, A., Naz, T., et al., (2020) Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba). Clinical phytoscience, 6, pp.1-10.
Comments (0)