Gastrointestinal stromal tumors (GISTs) are typically solitary, with rare instances of multiple occurrences (1). Existing literature has primarily associated the occurrence of multiple GISTs with specific scenarios, including individuals under 30 years of age (2), familial GISTs (hereditary GISTs) (3), neurofibromatosis type 1 (NF1) (4), and Carney’s triad and Carney-Stratakis syndrome (5, 6). Pediatric GISTs and the Carney triad and Carney-Stratakis syndrome, in particular, have frequently presented as multiple GISTs (2, 5, 6), while NFl and familial GIST syndrome often involve numerous tumors in one or more parts of the digestive tract, accompanied by hyperplasia of the diffuse interstitial cells of Cajal. The small intestine is a common site for NFl, while familial GISTs often manifest as multiple primary tumors at multiple sites (3, 4). These diseases exhibit unique clinical characteristics owing to their specific pathogenesis and medical history, facilitating clinical diagnosis. Primary multiple sporadic GISTs are rare and have only recently been confirmed (7, 8). These multiple primary tumors can simultaneously appear in the same or different parts of the same patient (stomach, intestine, or peritoneum), each displaying different KIT or PDGFRA mutation types (9). Herein, we report a case of primary multiple sporadic GISTs revealed by dual-time point positron emission tomography (PET) with 18F-labeled fluoro-2-deoxyglucose (18F-FDG) and computed tomography (18F-FDG PET/CT), with the purpose of exploring the diagnostic value of dual time point PET/CT in GIST and enhancing the understanding of this rare disease, providing valuable insights for both imaging diagnostic and clinical doctors.
2 Case descriptionA 64-year-old male patient suffered from recurrent abdominal pain and distension for 15 days who underwent upper abdominal CT scan in an external hospital 4 days ago and found abdominal mass. Now he came to our hospital for further diagnosis and treatment. Neither the patient nor his family had a history of tumors or genetic diseases. Routine physical examination revealed a slight elevation in serum Ca125 levels, with a value of 65.2 (reference value, <35). Other laboratory indicators and serum tumor marker values were within normal reference ranges. Whole abdominal CT revealed multiple soft tissue mass shadows in the abdominal cavity and retroperitoneum. Low-density cystic necrotic areas were observed in the mass. 18F-FDG PET/CT was recommended for the systematic evaluation of the patient’s condition. 18F-FDG PET/CT (Obtained after injection of 18F-FDG 60 min, Figure 1) revealed multiple soft tissue masses in the abdominal cavity and retroperitoneum, exhibiting heightened radioactive uptake, which were poorly demarcated from the surrounding structures, and the adjacent bowel was displaced by pressure (Figure 2). Furthermore, a delayed PET/CT scan at 180 min was performed to understand the delayed uptake of 18F-FDG by the mass over time and further determine the location of the mass and the relationship between the mass and the intestinal wall. The results indicated increased uptake of 18F-FDG in the mass, and presented migration of the mass in the lower abdomen, no longer retaining their initial position. Subsequently, the patient underwent exploratory laparoscopy, tumor resection, and complex intestinal adhesion lysis under general anesthesia. During the operation, it was found that the mass compressed the adjacent bowel without any direct connection. Therefore, it was considered that the mass may have originated from the mesentery. The lumps underwent pathological examination following the surgery. Hematoxylin and eosin staining revealed the presence of uniformly dispersed fusiform cells (as shown in Figure 3). Immunohistochemical results showed that the tumor positively expressed DOG-1, CD117, DESMIN, CK, CD34, S100, SMA, and vimentin, while it was negative for human melanoma black 45, CD56, NSE, and Bcl-2. Based on these findings, a diagnosis of GISTs (mitotic figure: 5-11/50 high power fields) was established. Subsequently, further genetic testing was conducted on these excised tumors, which revealed different KIT and PDGFRA mutation patterns, indicating a polyclonal origin. Consequently, the patient was diagnosed with multiple sporadic GISTs. After surgery, the patient underwent a 5-day anti-inflammatory treatment and was discharged without further chemotherapy. Subsequently, the patient underwent a 30-month follow-up, during which they remained healthy and alive.
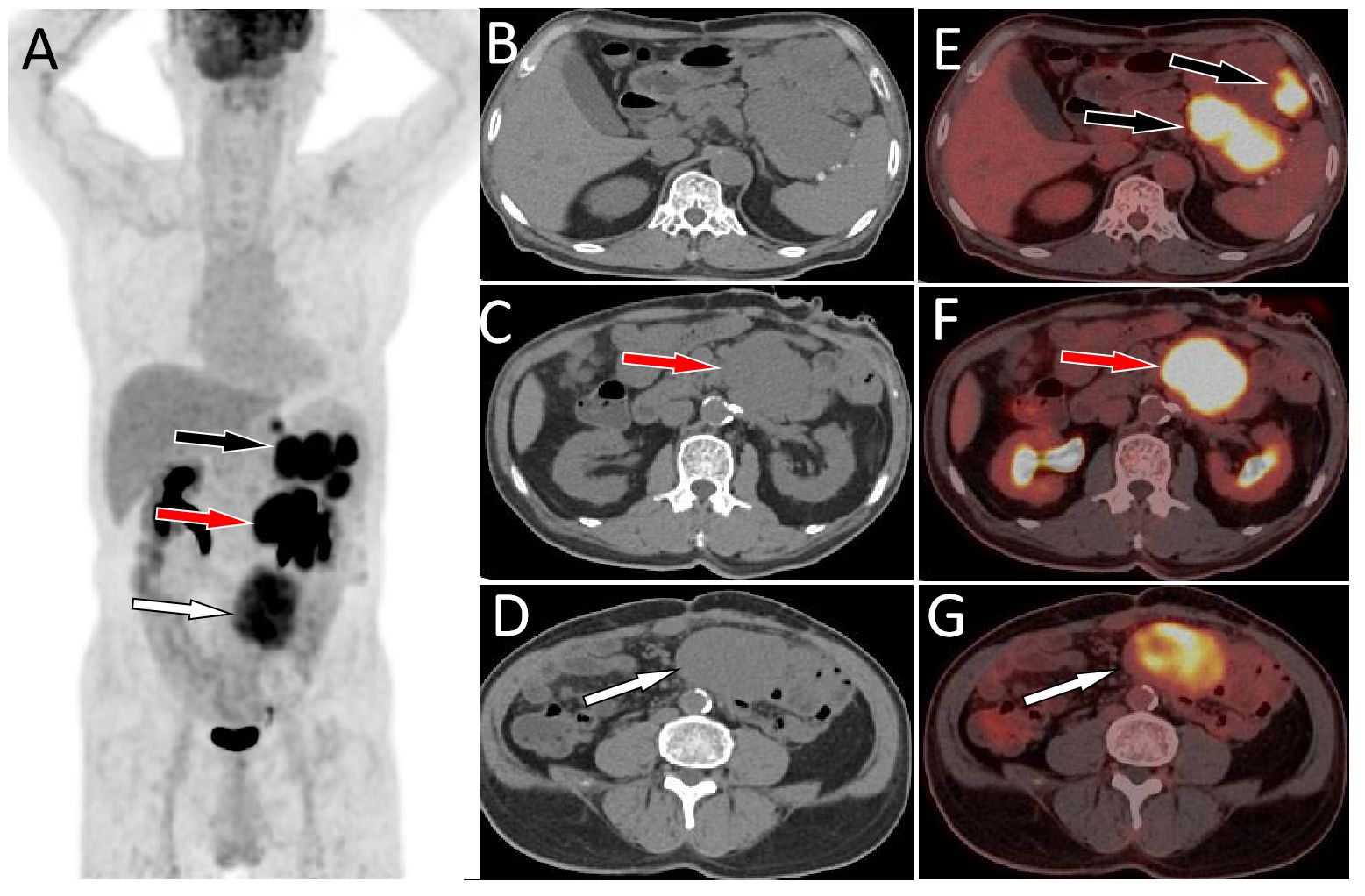
Figure 1 MIP image (A) acquired 60 min after injection showed that large intensity uptake in the left upper abdomen with SUVmax of 13.2 (black arrow), 14.6 (red arrow) and moderate radioactive uptake overlapping the spine with SUVmax of 9.1 (white arrow). On axial CT (B-D) and PET/CT fusion (E-G) images, the mass with high radioactivity uptake was located in the spleen-gastric space (black arrow), left retroperitoneum (red arrow), and right retroperitoneum (white arrow). Notes: MIP, maximum intensity projection; CT, computed tomography; PET, positron emission tomography; 18F-FDG, 18F-labeled fluoro-2-deoxyglucose; SUVmax, maximum standard uptake value.
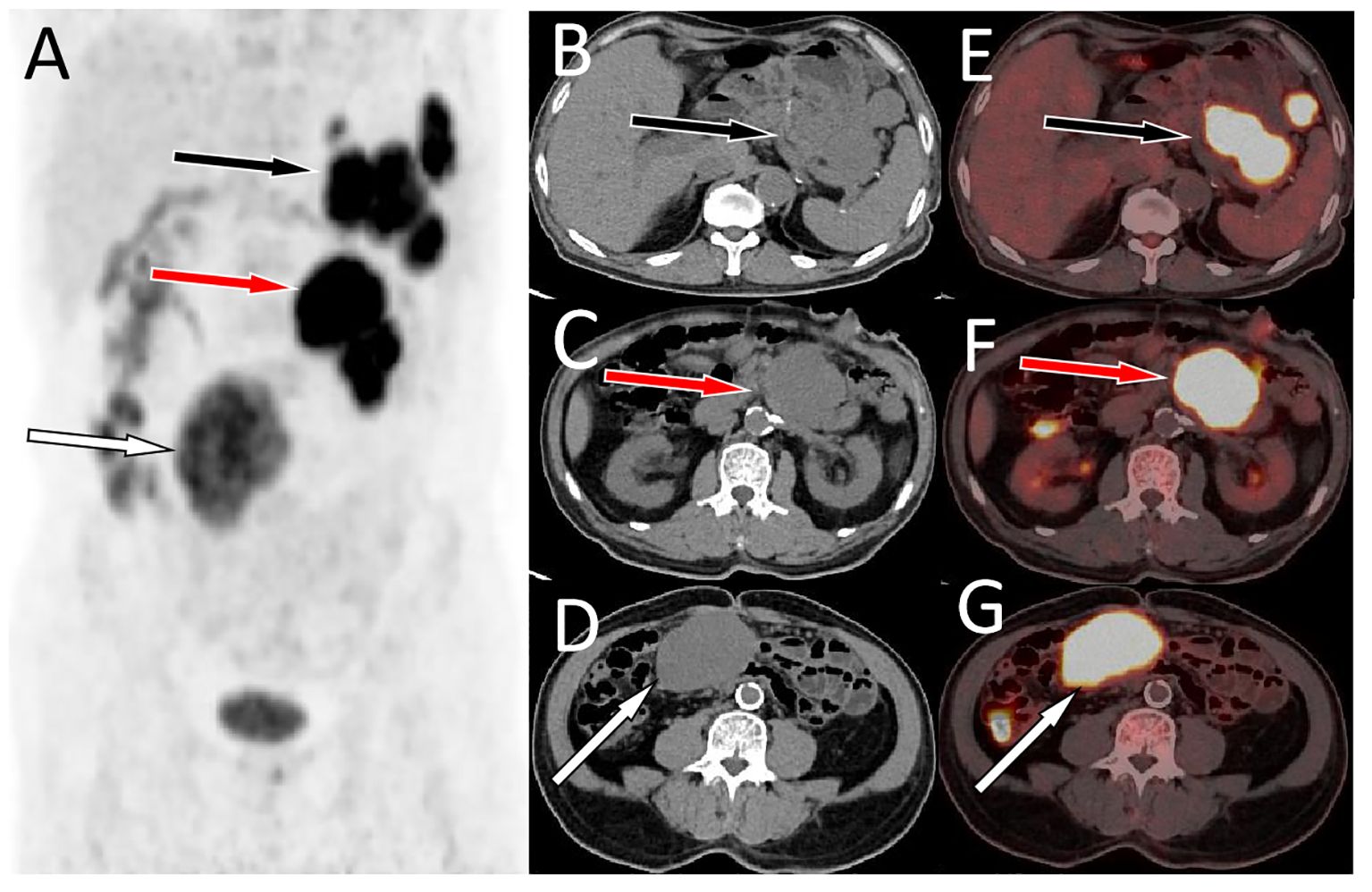
Figure 2 A delayed PET/CT scan at 180 min (A, MIP) was acquired, axial CT (B-D) and PET/CT fusion (E-G) showed a further increase in 18F-FDG radioactivity in the abovementioned lumps, with SUVmax of 16.0, 17.1, and 16.7 from top to bottom, respectively. Furthermore, the location of the lowest mass shifted from the initial left side of the spine to the right side of the spine (G, I, white arrows).Notes: MIP, maximum intensity projection; CT, computed tomography; PET, positron emission tomography; 18F-FDG, 18F-labeled fluoro-2-deoxyglucose; SUVmax, maximum standard uptake value.
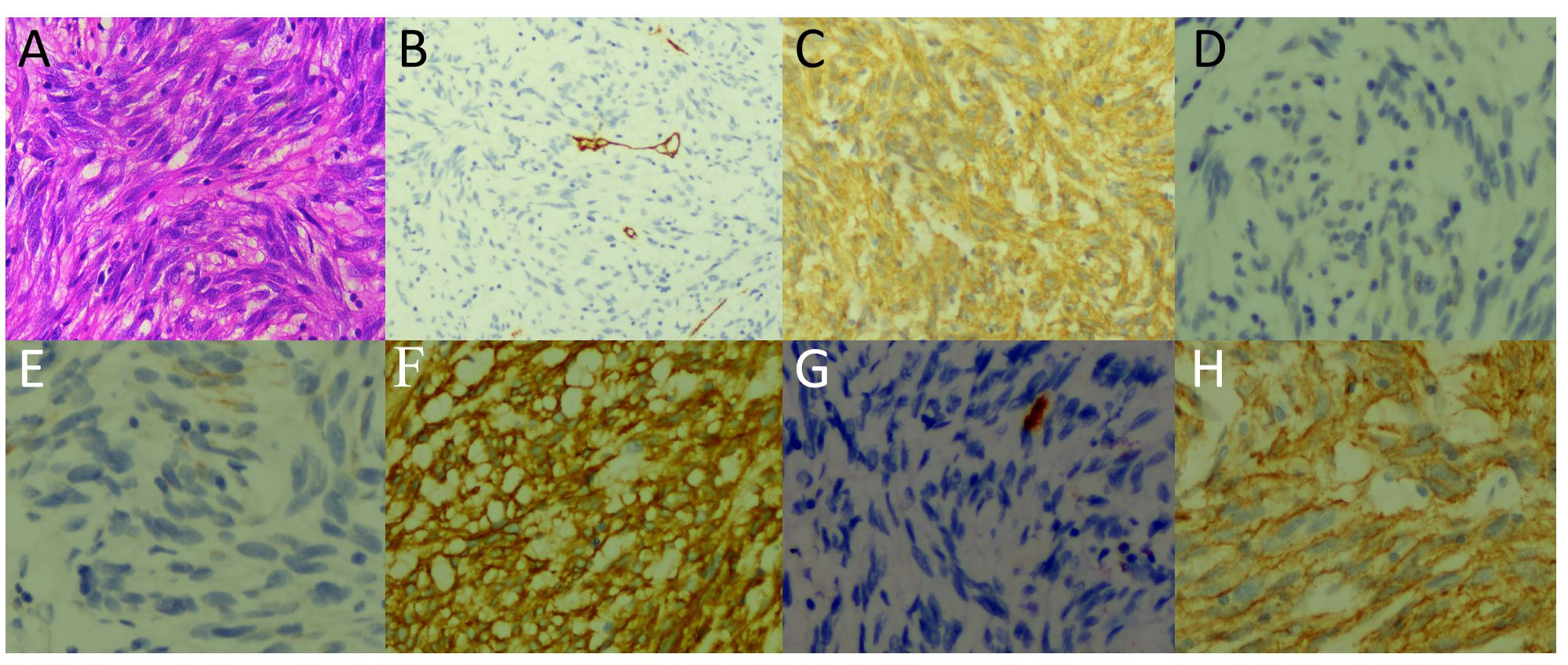
Figure 3 Hematoxylin and eosin staining showed uniformly dispersed fusiform cells (A). Immunohistochemical results showed that the tumor expressed CD34 (B), CD117 (C), DESMIN (D), CK (E), DOG-1 (F), S100 (G), and SMA (H).
3 DiscussionGIST are the most common stromal tumors in the gastrointestinal tract and can occur at various sites along this tract, with the stomach and small intestine being the most frequent locations. It is worth noting that certain GISTs may originate outside the gastrointestinal tract, including the omentum, mesentery, and retroperitoneum (10). Typically, GISTs occur as solitary entities, however, in rare instances, they may occur as multiple tumors distributed across one or more organs. Initially, the presence of multiple GISTs was primarily associated with familial GISTs, children, or specific tumor syndromes, such as NF1, Carney’s triad, and Carney-Stratakis syndrome (11). Furthermore, regardless of their number, size, or location, the occurrence of multiple GISTs is often considered tumor recurrence or metastasis. In 2007, Kang et al. examined five patients with sporadic multiple GISTs and identified different KIT gene mutation types (VVEEIN559-564del, V559, and V560 del) in distinct masses within the same patient. Each mass exhibited distinct cellular morphological and immunohistochemical characteristics, thereby proving the existence of sporadic multiple GISTs (7). Subsequently, Haller et al. conducted genetic testing on four patients with multiple GISTs and found that each tumor within the same patient had different KIT or PDGFRA mutation types, further confirming that these multiple GISTs were primary lesions (8). Multiple sporadic GISTs occurring outside of familial and symptomatic syndromes have been rarely documented and tend to occur in middle-aged and older adults, with a slightly higher incidence in men than in women (approximately 1.3:1) (12, 13). The most common clinical manifestations associated with multiple sporadic GISTs include abdominal pain, bloating, and intestinal obstruction resulting from compression of adjacent intestines by lumps (14). Here, we reported the case of a 64 year old male patient with no history of tumors or genetic diseases. The patient sought medical assistance for abdominal pain and bloating, which were consistent with the diagnosis and epidemiology of multiple sporadic GISTs.
The CT and magnetic resonance imaging (MRI) features of GIST, including large, circular, or lobulated soft tissue masses prone to cyst formation or necrosis in the center, and nonuniform delayed enhancement, have been reported in previous studies (15–17). Compared with traditional imaging techniques, PET/CT offers distinct advantages as it can simultaneously provide anatomical and metabolic information of lesions. Additionally, 18F-FDG PET/CT has been widely used for staging, re-staging, and monitoring the treatment response of GISTs (18, 19). The 18F-FDG PET/CT findings of GISTs correlate with their risk grade, with high-risk GISTs exhibiting a higher maximum standard uptake value (SUVmax) than that of medium-to low-risk GISTs (20). Furthermore, a previous study demonstrated a positive correlation between the SUVmax of 18F-FDG PET/CT and the Ki-67 index, tumor size and mitotic image. The sensitivity and specificity of SUVmax in predicting the risk grade were 85.7% and 94.7%, respectively. However, due to the rarity of multiple sporadic GISTs, the PET/CT findings have been less comprehensively documented in the literature. The case presented here showed multiple abdominal masses with unclear boundaries between adjacent structures. PET/CT revealed multiple lesions with varying degrees of uneven metabolic abnormalities in the abdomen and pelvis.
Multiple sporadic GISTs are rare, which should be differentiated from metastatic tumors, Castleman disease (CD), inflammatory myofibroblast tumor (IMT), and lymphoma (21). Metastases have a history of primary disease and varying degrees of radioactive uptake on PET/CT, according to the characteristics of the primary tumor. Most primary tumors can be visualized using PET/CT. The biological behaviors of IMT and CD are typically benign or tend to exhibit benign traits, featuring imaging characteristics akin to those of benign tumors, often presenting spherical soft tissue shadows with clear boundaries. The maximum standard uptake value of these tumors in PET/CT imaging is significantly lower compared to that of GISTs (22–24). Lymphoma, characterized by relatively uniform mass density and unclear boundaries with the adjacent gastrointestinal wall, is rarely necrotic and is often accompanied by multiple lymph node enlargements in the small and large curvatures of the stomach, abdominal trunk, or mesentery. Floating vascular shadows can be seen within the lesion, with a high uptake of 18F-FDG and an SUVmax >20 (25).
According to tumor cell morphology, GISTs can be divided into spindle, epithelioid, and mixed types (11). Distinguishing GISTs from other gastrointestinal tumors, such as non-GIST sarcomas and sarcomatoid carcinomas, based solely on tumor cytology can be challenging. Immunohistochemical testing can be used to diagnose suspected GISTs, among which CD117 and DOG-1 are the most important immunohistochemical detection indicators with high sensitivity and specificity. Moreover, the vast majority of GISTs positively express CD117 (95%) and DOG-l (98%) (26, 27). In addition to meeting the histopathological criteria for GISTs, the diagnosis of multiple sporadic GISTs also requires excluding familial GISTs, pediatric cases, or specific tumor syndromes such as NFl, Carney syndrome, and Carney-Stratakis syndrome. In the present case, spindle-shaped tumor cells were observed on microscopic examination, whose immunohistochemistry showed positive expression of CD117 and DOG-l; the patient had no evidence of familial GISTs or tumor syndromes. This aligns with the diagnosis of multiple sporadic GISTs. From a clinical perspective, diagnosing primary multiple sporadic GISTs is often more challenging than diagnosing GISTs accompanied by related tumor syndromes or metastasized disseminated GISTs. Nevertheless, accurate diagnosis significantly impacts disease staging and consequently influences treatment strategies. Therefore, to reduce the occurrence of clinical misdiagnosis, we have developed a diagnostic flowchart for GISTs, as shown in Figure 4.
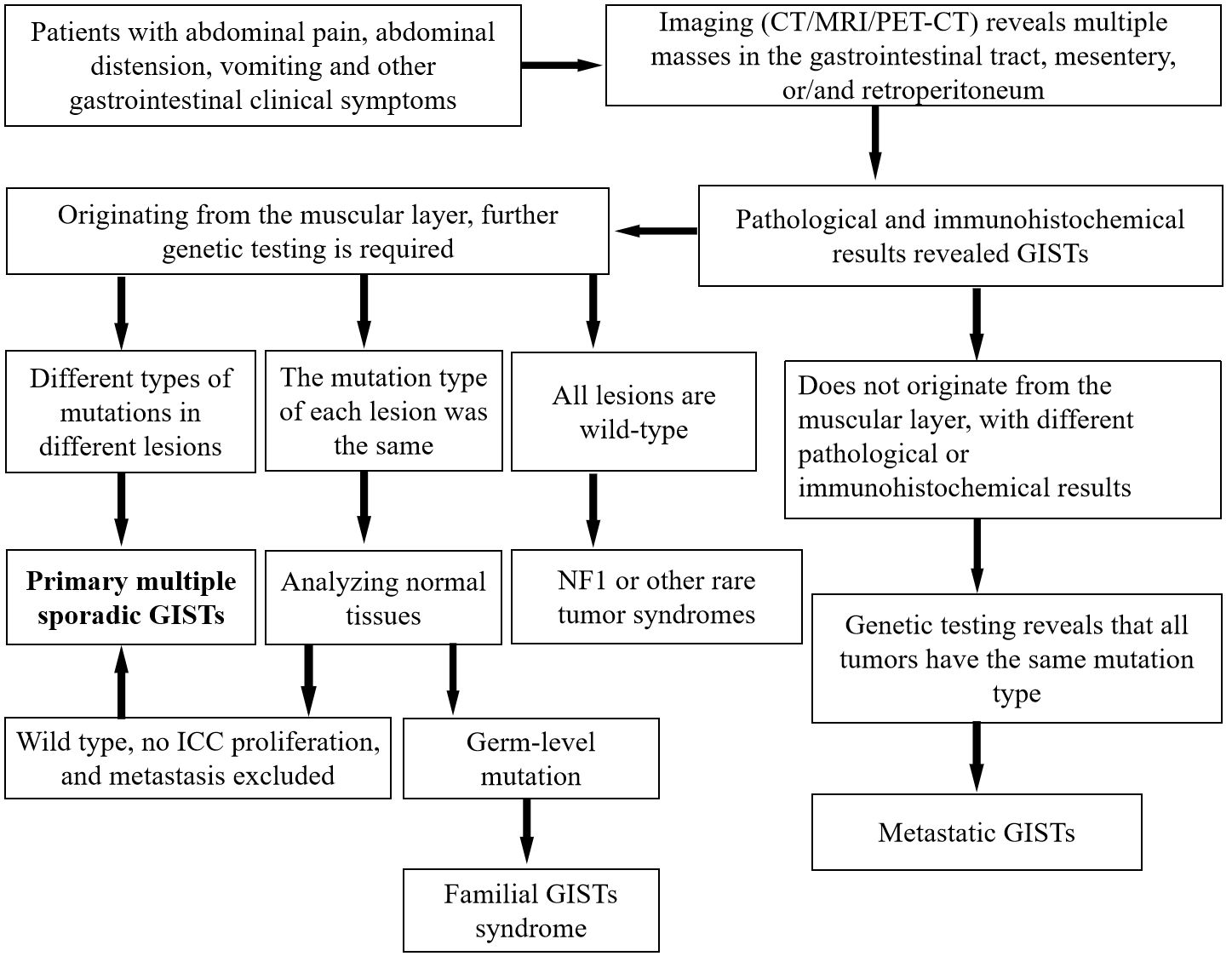
Figure 4 General diagnostic flowchart for multiple GISTs. CT, computed tomography; GISTs, gastrointestinal stromal tumors; ICC, interstitial cells of Cajal; MRI, magnetic resonance imaging; PET, positron emission tomography.
Surgical resection is the preferred treatment for multiple sporadic GISTs, and the scope of surgical resection is determined based on the site, size, nature of the tumor, and the patient’s overall systemic condition (28). In patients with advanced stages or metastasis, imatinib can be used to alleviate the progression of the condition (11). Multiple sporadic GISTs can exhibit varying malignant potentials, ranging from benign to lethal malignant tumors. As a result, their prognosis is comparable to that of isolated GISTs. A previous extensive clinical study showed that 5-year and 15-year disease-free survival rates of GISTs were 70.5% and 59.9%, respectively (29). Our patient underwent tumor removal surgery without any additional treatments. After a 30-month follow up, the patient remains in good health and is still alive.
4 ConclusionIn conclusion, our study has shed light on the important role of PET/CT in the diagnosis of multiple sporadic GISTs, especially those originating outside the gastrointestinal tract. The migration of the mass in the lower abdomen was observed on dual time point PET/CT, and an increase in radiation uptake was observed 3 hours later compared to 1 hour after 18F-FDG injection, which is an important diagnostic indicator. This finding holds promising clinical implications, potentially influencing the early diagnosis and management of patients with GIST. The migration of the mass in the lower abdomen and differential uptake in dual-time-point PET/CT could provide valuable findings for healthcare professionals in making more accurate and timely diagnostic decisions.
Data availability statementThe original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statementEthical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the [patients/participants OR patients/participants legal guardian/next of kin] was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributionsCL: Conceptualization, Data curation, Validation, Writing – original draft. WL: Methodology, Software, Validation, Writing – original draft. MS: Investigation, Project administration, Supervision, Writing – original draft. PW: Investigation, Supervision, Validation, Writing – review & editing. XH: Conceptualization, Formal analysis, Methodology, Resources, Writing – review & editing.
FundingThe author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is supported by Zun City Kehe (Grant Number: HZ-2023-284).
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References2. Benesch M, Wardelmann E, Ferrari A, Brennan B, Verschuur A. Gastrointestinal stromal tumors (GIST) in children and adolescents: A comprehensive review of the current literature. Pediatr Blood Cancer. (2009) 53:1171–9. doi: 10.1002/pbc.22123
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Li FP, Fletcher JA, Heinrich MC, Garber JE, Sallan SE, Curiel-Lewandrowski C, et al. Familial gastrointestinal stromal tumor syndrome: phenotypic and molecular features in a kindred. J Clin Oncol. (2005) 23:2735–43. doi: 10.1200/JCO.2005.06.009
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Yamamoto H, Tobo T, Nakamori M, Imamura M, Kojima A, Oda Y, et al. Neurofibromatosis type 1-related gastrointestinal stromal tumors: a special reference to loss of heterozygosity at 14q and 22q. J Cancer Res Clin Oncol. (2009) 135:791–8. doi: 10.1007/s00432-008-0514-z
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet. (2002) 108:132–9. doi: 10.1002/ajmg.10235
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Pasini B, McWhinney SR, Bei T, Matyakhina L, Stergiopoulos S, Muchow M, et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet. (2008) 16:79–88. doi: 10.1038/sj.ejhg.5201904
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Kang DY, Park CK, Choi JS, Jin SY, Kim HJ, Joo M, et al. Multiple gastrointestinal stromal tumors: Clinicopathologic and genetic analysis of 12 patients. Am J Surg Pathol. (2007) 31:224–32. doi: 10.1097/01.pas.0000213318.66800.94
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Haller F, Schulten HJ, Armbrust T, Langer C, Gunawan B, Füzesi L. Multicentric sporadic gastrointestinal stromal tumors (GISTs) of the stomach with distinct clonal origin: differential diagnosis to familial and syndromal GIST variants and peritoneal metastasis. Am J Surg Pathol. (2007) 31:933–7. doi: 10.1097/01.pas.0000213440.78407.27
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Gasparotto D, Rossi S, Bearzi I, Doglioni C, Marzotto A, Hornick JL, et al. Multiple primary sporadic gastrointestinal stromal tumors in the adult: an underestimated entity. Clin Cancer Res. (2008) 14:5715–21. doi: 10.1158/1078-0432.CCR-08-0622
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Zhu J, Yang Z, Tang G, Wang Z. Extragastrointestinal stromal tumors: Computed tomography and magnetic resonance imaging findings. Oncol Lett. (2015) 9:201–8. doi: 10.3892/ol.2014.2705
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Li K, Tjhoi W, Shou C, Yang W, Zhang Q, Liu X, et al. Multiple gastrointestinal stromal tumors: analysis of clinicopathologic characteristics and prognosis of 20 patients. Cancer Manag Res. (2019) 11:7031–8. doi: 10.2147/CMAR.S197560
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Agaimy A, Dirnhofer S, Wünsch PH, Terracciano LM, Tornillo L, Bihl MP. Multiple sporadic gastrointestinal stromal tumors (GISTs) of the proximal stomach are caused by different somatic KIT mutations suggesting a field effect. Am J Surg Pathol. (2008) 32:1553–9. doi: 10.1097/PAS.0b013e31817587ea
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Agaimy A, Märkl B, Arnholdt H, Wünsch PH, Terracciano LM, Dirnhofer S, et al. Multiple sporadic gastrointestinal stromal tumours arising at different gastrointestinal sites: pattern of involvement of the muscularis propria as a clue to independent primary GISTs. Virchows Arch. (2009) 455:101–8. doi: 10.1007/s00428-009-0803-1
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Kim KH, Nelson SD, Kim DH, Choi KU, Kim SJ, Min KW, et al. Diagnostic relevance of overexpressions of PKC-θ and DOG-1 and KIT/PDGFRA gene mutations in extragastrointestinal stromal tumors: a Korean six-centers study of 28 cases. Anticancer Res. (2012) 32:923–37.
PubMed Abstract | Google Scholar
15. Sandrasegaran K, Rajesh A, Rushing DA, Rydberg J, Akisik FM, Henley JD. Gastrointestinal stromal tumors: CT and MRI findings. Eur Radiol. (2005) 15:1407–14. doi: 10.1007/s00330-005-2647-7
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Du H, Ning L, Li S, Lou X, Chen H, Hu F, et al. Diagnosis and treatment of duodenal gastrointestinal stromal tumors. Clin Transl Gastroenterol. (2020) 11:e00156. doi: 10.14309/ctg.0000000000000156
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Inoue A, Ota S, Yamasaki M, Batsaikhan B, Furukawa A, Watanabe Y. Gastrointestinal stromal tumors: a comprehensive radiological review. Jpn J Radiol. (2022) 40:1105–20. doi: 10.1007/s11604-022-01305-x
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Altini C, Mammucci P, Pisani AR, D'Alò C, Sardaro A, Rubini D, et al. (18)F-FDG PET/CT in GIST treatment response evaluation beyond Imatinib. Hell J Nucl Med. (2021) 24:239–46. doi: 10.1967/s002449912407
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2018) 29:iv267. doi: 10.1093/annonc/mdy320
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Otomi Y, Otsuka H, Morita N, Terazawa K, Furutani K, Harada M, et al. Relationship between FDG uptake and the pathological risk category in gastrointestinal stromal tumors. J Med Invest. (2010) 57:270–4. doi: 10.2152/jmi.57.270
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Xiao JX, Ye J, Huang SS, Guo CM, Su M, Wang D. A rare case of multiple primary extragastrointestinal stromal tumors in the abdominopelvic cavity detected by (18)F-FDG PET/CT. Hell J Nucl Med. (2020) 23:346–8. doi: 10.1967/s002449912212
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Díaz Silván A, Allende Riera A, Cabello García D, Vilahomat Hernández O, Martínez Gimeno E. Inflammatory myofibroblastic tumor. (18)F-FDG PET/CT findings. Rev Esp Med Nucl Imagen Mol (Engl Ed). (2019) 38:190–1. doi: 10.1016/j.remn.2018.06.004
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Dong A, Wang Y, Dong H, Gong J, Cheng C, Zuo C, et al. Inflammatory myofibroblastic tumor: FDG PET/CT findings with pathologic correlation. Clin Nucl Med. (2014) 39:113–21. doi: 10.1097/RLU.0b013e3182952caa
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Jiang Y, Hou G, Zhu Z, Huo L, Li F, Cheng W. 18F-FDG PET/CT imaging features of patients with multicentric Castleman disease. Nucl Med Commun. (2021) 42:833–8. doi: 10.1097/MNM.0000000000001404
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Kahle XU, Montes de Jesus FM, Kwee TC, van Meerten T, Diepstra A, Rosati S, et al. Relationship between semiquantitative (18)F-fluorodeoxyglucose positron emission tomography metrics and necrosis in classical Hodgkin lymphoma. Sci Rep. (2019) 9:11073. doi: 10.1038/s41598-019-47453-5
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Huss S, Pasternack H, Ihle MA, Merkelbach-Bruse S, Heitkötter B, Hartmann W, et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal GISTs are rare events. Hum Pathol. (2017) 62:206–14. doi: 10.1016/j.humpath.2017.01.005
PubMed Abstract | CrossRef Full Text | Google Scholar
28. Bachet JB, Landi B, Laurent-Puig P, Italiano A, Le Cesne A, Lévy P, et al. Diagnosis, prognosis and treatment of patients with gastrointestinal stromal tumour (GIST) and germline mutation of KIT exon 13. Eur J Cancer. (2013) 49:2531–41. doi: 10.1016/j.ejca.2013.04.005
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. (2012) 13:265–74. doi: 10.1016/S1470-2045(11)70299-6
留言 (0)