Air pollution significantly impact lung cancer progression, but there is a lack of a comprehensive molecular characterization of clinical samples associated with air pollution. Here, we performed a proteogenomic analysis of lung adenocarcinoma (LUAD) in 169 female never-smokers from the Xuanwei area (XWLC cohort), where coal smoke is the primary contributor to the high lung cancer incidence. Genomic mutation analysis revealed XWLC as a distinct subtype of LUAD separate from cases associated with smoking or endogenous factors. Mutational signature analysis suggested that Benzo[a]pyrene (BaP) is the major risk factor in XWLC. The BaP-induced mutation hotspot, EGFR-G719X, was present in 20% of XWLC which endowed XWLC with elevated MAPK pathway activations and worse outcomes compared to common EGFR mutations. Multi-omics clustering of XWLC identified four clinically relevant subtypes. These subgroups exhibited distinct features in biological processes, genetic alterations, metabolism demands, immune landscape, tumor microbiota composition and radiomic features. Finally, MAD1 and TPRN were identified as novel potential therapeutic targets in XWLC. Our study provides a valuable resource for researchers and clinicians to explore prevention and treatment strategies for air-pollution-associated lung cancers.
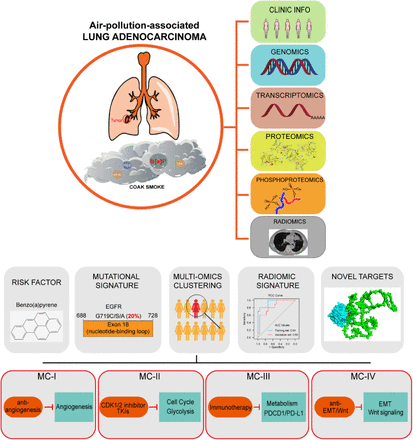

Graphical Abstract
Highlights
We conducted comprehensive multi-omic profiling of air-pollution-associated LUAD.
Our study revealed the significant roles of the air pollutant BaP and its induced hot mutation G719X in lung cancer progression.
Multi-omic clustering enabled the identification of personalized therapeutic strategies.
Through mutation-informed interface analysis, we identified novel targets for therapeutic intervention.
Competing Interest StatementThe authors have declared no competing interest.
Funding StatementThis work was supported by National Natural Science Foundation (Nos. 82273501, 81960322, 82160343, 82060519, 81860171); Yunnan young and middle-aged academic and technical leaders Reserve Talents Project (No. 202005AC160048); Yunnan basic research program (Nos.202101AZ070001-002, 202001AY070001-277, 2019FE001(-236)); Medical Reserve Personnel Training Program of Yunnan Provincial Health Commission(No. H-2018097); Famous Doctor Special Project of Ten Thousand People Plan of Yunnan Province(Nos.CZ0096, YNWR-MY-2020-095); Medical Leading Talents Training Program of Yunnan Provincial Health Commission (No.L-2019028).
Author DeclarationsI confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
Yes
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
The study protocol was reviewed and approved by the ethical committees of Yunnan Cancer Hospital & The Third Affiliated Hospital of Kunming Medical University (KYCS2022067) and conformed to the ethical standards for medical research involving human subjects, as laid out in the 1964 Declaration of Helsinki and its later amendments.Written informed consent was obtained from the patient and the ethical committees of Yunnan Cancer Hospital & The Third Affiliated Hospital of Kunming Medical University for publication of this study.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
Yes
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
Yes
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Yes
Data AvailabilityRaw sequencing data have been deposited in the Genome Sequence Archive for Human (GSA-Human, https://ngdc.cncb.ac.cn/gsa-human/) under accession codes HRA000124, HRA001481 and HRA001482. Proteomics and phosphoproteomics data have been deposited in the Open Archive for Miscellaneous Data (OMIX, https://ngdc.cncb.ac.cn/omix/) under accession codes OMIX001292. The raw lung CT images used in this paper are available from OMIX under accession codes OMIX002491.
留言 (0)