Tuberculosis (TB) is a leading cause of death worldwide. Mycobacterium tuberculosis (MTb) has infected approximately one-fourth of the world population with 1.7 billion estimated cases of infection.[1] Due to the high burden of TB in China, it has been classified as a notifiable disease.[2]
The diagnosis of pulmonary lesions caused by endobronchial TB (EBTB) is conventionally based on sample retrieval using the fiberoptic bronchoscopy procedure. The brush material with or without alveolar lavage fluid is sent separately for laboratory examination and cytological assessment, whereas the biopsy sample is submitted for histopathological examinations. However, the cytological features of brushing materials obtained in EBTB have rarely been reported in the English literature.[3] Practically, some morphological changes, especially squamous reactive atypia, may confuse cytologists and can be difficult to distinguish from the well-differentiated squamous cell carcinoma. Being one of the primary conditions in the differential diagnosis of TB, lung carcinoma is the leading cause of cancer-related death among men and the second-leading cause of cancer-related death among women worldwide.[4] Moreover, China has the highest incidence, mortality rate, and disability-adjusted life years of lung carcinoma.[5,6] Therefore, a review of the bronchoscopy performance, pathological examinations, and laboratory tests to summarize the morphologic features, diagnostic pitfalls, and dilemmas would help in reaching a final pathological diagnosis.
At our institution, EBTB diagnosis based on tissue biopsies and brush cytology specimens are conducted separately by a respiratory pathologist and cytologist, respectively. Therefore, this study aimed to explore the similarities and discordance of the two morphological examinations and determine the reasons for “atypical diagnosis” in the assessments.
MATERIAL AND METHODS Study population and data collectionAll patients diagnosed as EBTB between July 2017 and June 2020 at the First Affiliated Hospital, Hengyang Medical School, University of South China were enrolled and studied retrospectively. The inclusion criteria were as follows: (1) Visible endobronchial lesions on bronchoscopy; (2) both brushings and paired forceps biopsy specimens were obtained during the same bronchoscopy procedure; (3) positive results in at least one of the following examinations: Acid-fast bacilli stain (AFB) of the sputum smear or brushing materials or bronchoalveolar lavage, auramine-rhodamine (A-R) stain of the biopsy specimens, detection of TB bacteria DNA (TB-DNA) by polymerase chain reaction using sputum or bronchial perfusate, T-cell spot (T-spot), and typical TB pathologic changes on cytology or bronchoscopy biopsy; and (4) clinical regression of endobronchial lesions after anti-TB therapy. In addition, two cases that had concurrent lung carcinomas were also included in the study. The clinical features, endobronchial findings, laboratory examination results, cytological assessment, and paired biopsies of all the patients were collected. The exclusion criteria included the following: (1) Prior empirical anti-TB treatment before bronchoscopic examination and (2) existence of missing value during data collection. Therefore, 72 cytologicalhistological pairs were included in our study.
Sample processingThe brushings were fixed in a cell storage solution (Guangzhou Anbiping Medical Company Technology Co., Ltd., Guangzhou, China) and processed using a sedimentation cell prep plus liquid-based cytology processor under the liquid-based preparation (LBP) system (LBP-2601, Guangzhou Anbiping Medical Company Technology Co., Ltd.). All cells were automatically sedimented onto a glass slide, forming a diagnostic area with a diameter of 13 mm.[7] The area was stained using Papanicolaou stain. The biopsy tissue was embedded in paraffin, sliced into 3-μm-thick sections, and stained with hematoxylin and eosin.
Data analysisThe bronchoscopic features in the database were reviewed by a pulmonary specialist to identify the subtypes.[8] The histology and cytology specimens were reviewed separately by a respiratory pathologist and a cytologist, respectively. The detection rates of morphologic features such as metaplastic squamous cells, necrosis, epithelioid cells, and Langhans giant cells in both specimens were statistically analyzed using R language software (www.r-project.org; v4.0.5). McNemar’s Chi-square test with continuity correction was performed to assess the differences in the detection rates of the histological and cytological features. Results were considered statistically significant at P < 0.05.
RESULTSThis study included 42 female and 30 male patients, with an average age of 58.1 years. Two patients had comorbid carcinoma, and 47 of the remaining 70 patients were correctly diagnosed with TB through bronchoscopy. However, endoscopic assessments varied in the other 23 patients, of which nine were suspected to have TB or carcinoma, nine were considered to have non-specific inflammation, four were identified as carcinoma, and one was diagnosed with TB or fungal infection. Subtype identification based on bronchoscopic findings demonstrated inflammation infiltration in nine patients, ulceration necrosis in 27 patients, granulation hyperplasia in 16 patients, cicatrices stricture in 17 patients, and trachea bronchial malacia in one patient.
Table 1 shows the positive results of various laboratory examinations. AFB, A-R, TB-DNA, and T-spot were positive in 39, 26, 33, and 46 cases, respectively. As presented in Table 2, the detection rate of necrosis in the cytological assessments (90.3%) was significantly higher than that in the biopsy specimens (77.8%; P < 0.01) [Figure 1a]. In contrast, the detection rate of Langhans giant cells in brushing cytology (13.9%) was significantly lower than that in the biopsy specimens (38.9%; P < 0.01) [Figure 1b]. The detection rate of epithelioid cells in the cytological assessments (47.2%) showed no significant difference compared to that in the biopsy (61.1%; P > 0.05) [Figure 1c]. Either or both epithelioid cells and Langhans giant cells were defined as typical TB pathological changes, which showed higher sensitivity (66.7%) in biopsy histopathology than that in brushing cytology (48.61%). The specificity of the two methods was 100% due to the fact that other inflammatory diseases were not included in the study. Therefore, the positive predictive value of them was equal to sensitivity. Although metaplastic squamous cells were observed in 35/72 (48.6%) and 28/72 (38.9%) patients in the cytological assessments and histological evaluations, respectively [Figure 2a and b], the difference was not statistically significant (P > 0.05).
Table 1: Comparisons of positive results of various laboratory examinations.
AFB positive (n) A-R positive (n) TB-DNA positive (n) T-SPOT positive (n) AFB only 4 A-R only 2 TB-DNA only 4 T-SPOT only 7 AFB/TB-DNA 6 A-R/AFB 3 TB-DNA/AFB 6 T-SPOT/AFB 5 AFB/T-SPOT 5 A-R/TB-DNA 1 TB-DNA/T-SPOT 5 T-SPOT/TB-DNA 5 AFB/A-R 3 A-R/T-SPOT 7 TB-DNA/A-R 1 T-SPOT/A-R 7 AFB/TB-DNA/T-SPOT 10 A-R/AFB/TB-DNA 1 TB-DNA/AFB/T-SPOT 10 T-SPOT/AFB/TB-DNA 10 AFB/TB-DNA/A-R 1 A-R/TB-DNA/T-SPOT 2 TB-DNA/T-SPOT/A-R 2 T-SPOT/AFB/A-R 6 AFB/T-SPOT/A-R 6 A-R/AFB/T-SPOT 6 TB-DNA/AFB/A-R 1 T-SPOT/TB-DNA/A-R 2 AFB/TB-DNA/T-SPOT/A-R 4 A-R/AFB/TB-DNA/T-SPOT 4 TB-DNA/AFB /T-SPOT/A-R 4 T-SPOT/AFB/TB-DNA/A-R 4 Total 39 26 33 46Table 2: Comparisons of the detection rate of morphologic features in brushing cytology and biopsy pathology (n [%]).
Histology Metaplastic squamous cells Necrosis Epithelioid cells Langhans giant cells Positive Negative Total Positive Negative Total Positive Negative Total Positive Negative Total Cytology Positive (%) 23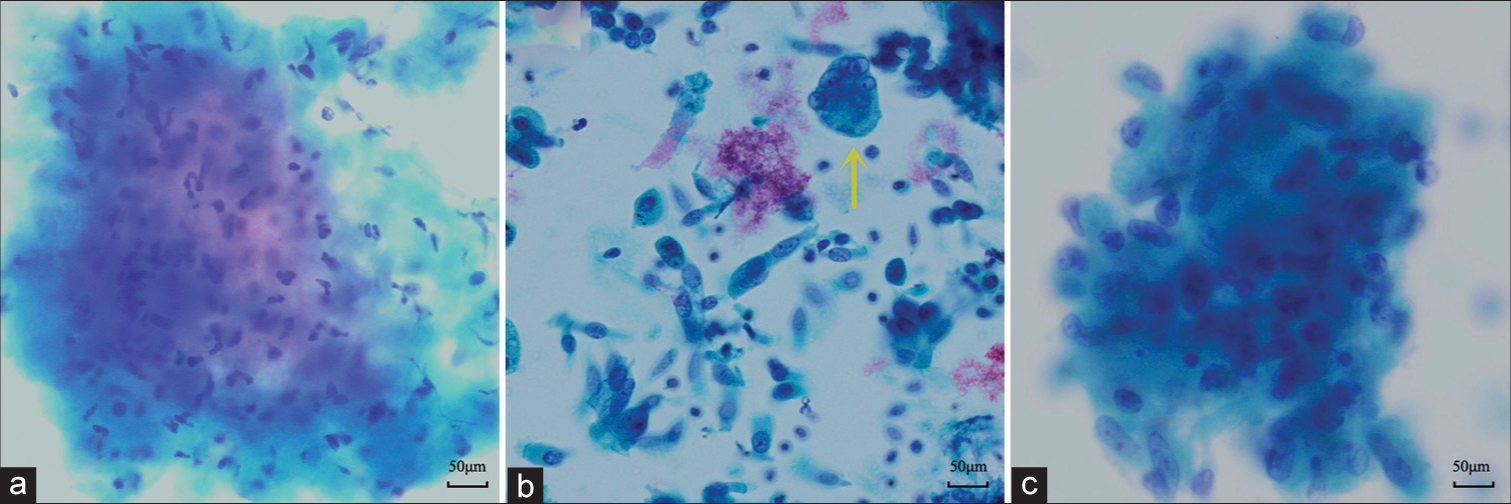
Export to PPT
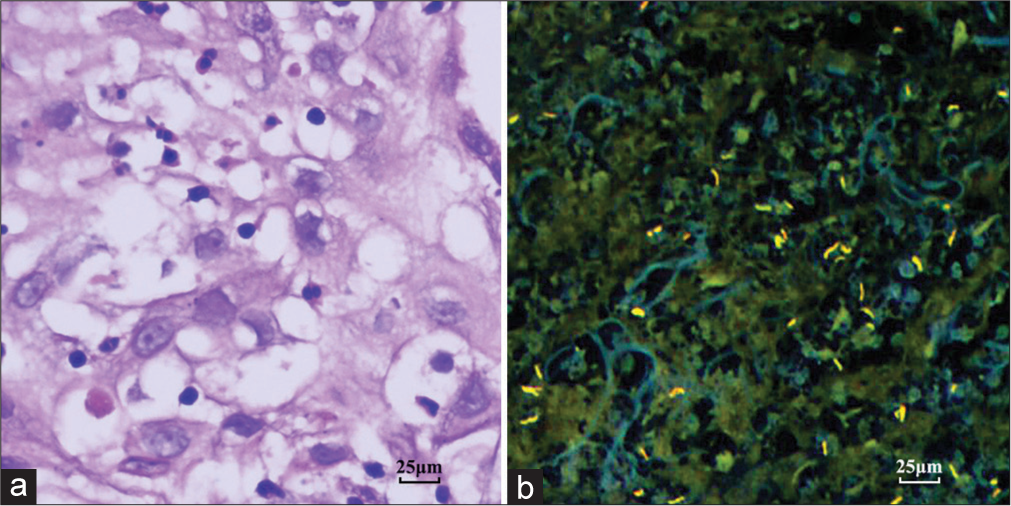
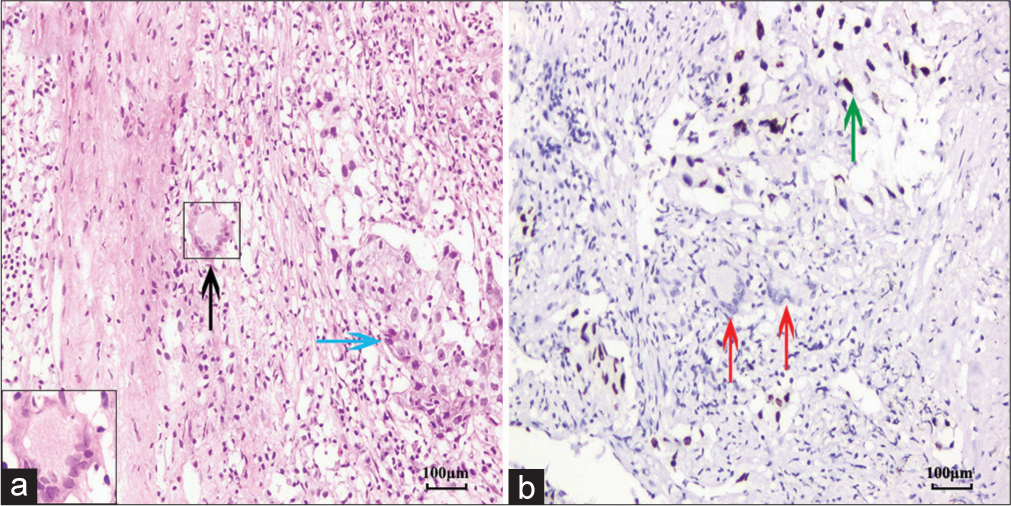
The contributing factors for the cytological diagnosis of “atypical cells” are summarized in Table 3. Besides, two patients with large amounts of short spindle cells could not be distinguished from spindle cell neoplasms, the other seven showed an atypical impression of metaplastic squamous cells, of which five were considered as reactive atypia and two were suspected as lung carcinomas. The reasons for the reactive atypia in the non-keratinizing squamous cells were as follows: Prominent nucleoli (n = 2) [Figure 2c and d], an enlarged nucleus with more than 2 times the volume of the surrounding nuclei (n = 1), and hyperchromasia (n = 2) [Figure 2e and f]. The two patients with suspicion of carcinomas showed some twisted or elongated keratinocytes in brushing cytology mimicking the keratinizing squamous cell carcinoma [Figure 2g]. Although the paired biopsy showed the continuous epithelial structure of metaplasia and reactive changes in most cases, scattered and distorted squamous cells were also encountered [Figure 2h]. Only one biopsy could not be excluded as well-differentiated squamous cell carcinoma because it showed a disorganized mass of distorted keratinocytes in the background of necrosis and scattered MTb [Figure 3a and b]. The lesion regressed during the follow-up antitubercular therapy. The comorbid malignant lesions in the two cases were confirmed by immunohistochemistry [Figure 4a and b]. One was adenocarcinoma and the other was squamous cell carcinoma.
Table 3: Review of the ten cases with diagnoses of “atypical cells.”
Case Endoscope impression Cytology diagnosis Biopsy diagnosis Contributing factors to “atypical cell” 1 TB Atypical cells, favor squamous reactive atypia TB Abrupt enlarged nucleus in metaplastic squamous cells 2 TB Atypical cells, favor squamous reactive atypia TB Metaplastic squamous cells showing slightly coarse chromatin granules 3 Non-special inflammation Atypical cells, favor squamous reactive atypia Chronic granulomatous inflammation Metaplastic squamous cells demonstrating prominent nucleoli 4 TB Atypical cells, favor squamous reactive atypia TB Metaplastic squamous cells showing slightly coarse chromatin granules 5 TB Atypical cells suspected as malignancy Necrosis Twisted or elongated keratinocytes showing atypia 6 CA or TB Atypical spindle cells, cannot exclude spindle cell neoplasm TB Aggregates of epithelioid histiocytes similar to neoplastic spindle cells 7 Non-special inflammation Atypical spindle cells, not exclude spindle cell neoplasm Chronic granulomatous inflammation Aggregates of epithelioid histiocytes similar to neoplastic spindle cells 8 CA Atypical cells suspected as malignancy Non-special inflammation with some uncertain cells Metaplastic squamous cells demonstrating prominent nucleoli 9 TB Atypical cells, favor of squamous reactive atypia TB Twisted or elongated keratinocytes showing atypia 10 TB Necrosis Atypical squamous nests cannot exclude squamous cell CA Twisted or elongated keratinocytes arranging in nests.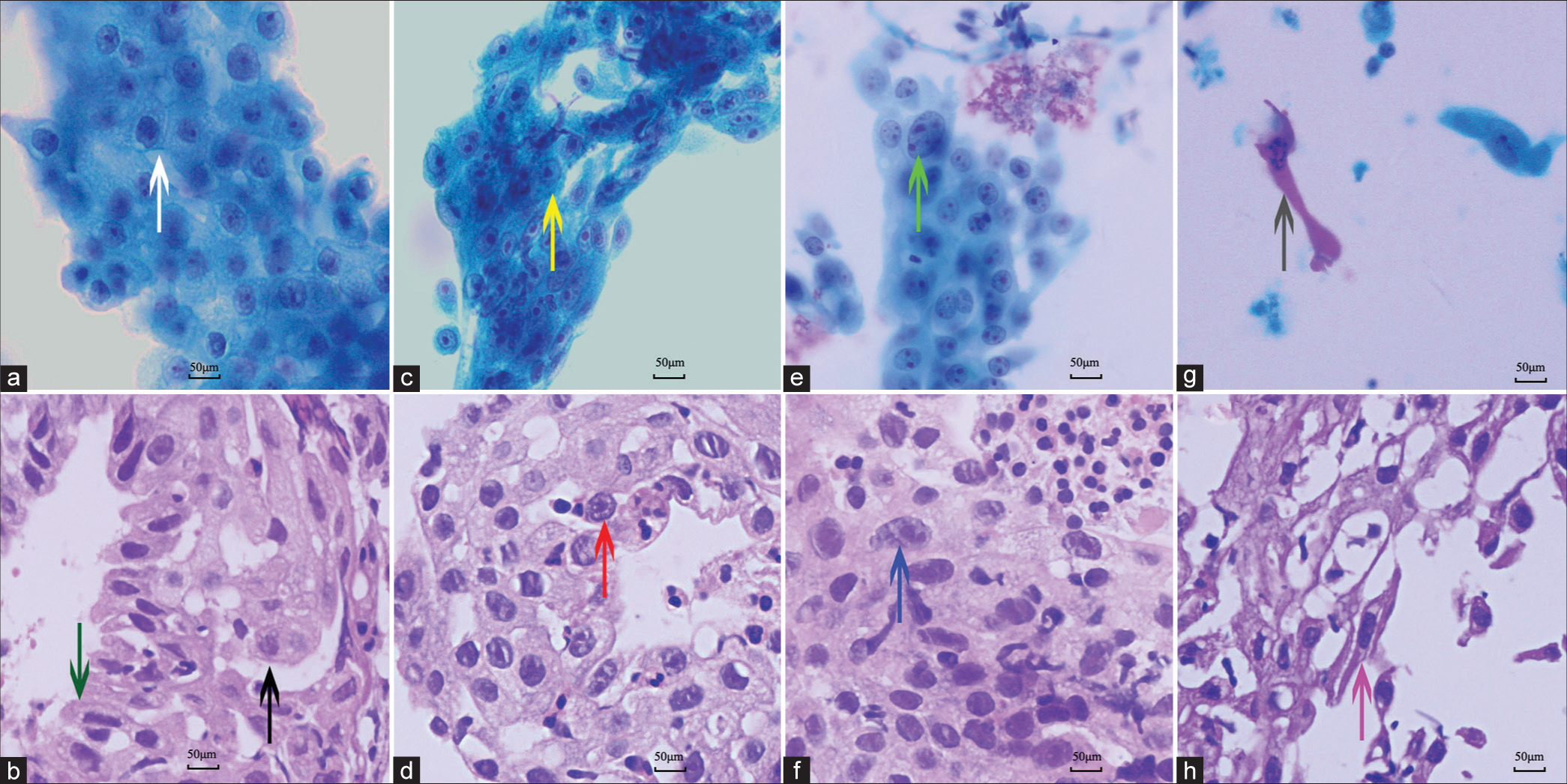
Export to PPT
Export to PPT
Export to PPT
DISCUSSIONTo the best of our knowledge, this is the first retrospective clinicopathological study of EBTB focusing on morphological characteristics. In line with some previous studies, EBTB was more likely to be associated with female patients.[9,10] Bronchoscopy is the most useful method to diagnose EBTB.[9,11] In fact, most patients in our study with indefinite imaging results underwent fiberoptic bronchoscopy examination for further evaluation. Although the final review of bronchoscopic findings demonstrated that the distribution of various subtypes was in accordance with other studies,[9] only 47 patients were initially diagnosed with EBTB. Based on the endoscopic findings, carcinomas were suspected in 13 patients, and malignancy was diagnosed in four of those patients. EBTB with the main characteristic of inflammatory infiltration is also easily inaccurately referred to as non-special inflammation and shows few typical TB morphologic features in biopsy histopathology. Mucosa hyperemia and edema are the main features of this subtype. Sometimes some granular nodules can be found under bronchoscopy. These findings emphasize the importance of distinguishing TB infectious from malignancies and other infectious diseases in subsequent morphological and laboratory examinations.[12]
EBTB also demonstrates reactive atypia on bronchial brushing cytology and biopsy pathology.[3] However, most cases can be correctly diagnosed by a series of auxiliary examinations such as AFB of the sputum smear, brushing materials, or bronchioalveolar lavage; A-R on biopsy specimen; Tb-DNA of sputum or bronchial perfusate; and T-spot.[13-15] Therefore, the morphological mimics seldom lead to eventual misdiagnoses. Nevertheless, the diagnosis of atypia may raise excessive concerns.
Atypia in cytology is mainly derived from morphological changes in squamous metaplasia. The normal responses of the columnar respiratory epithelium to long-standing stimulation lead to squamous metaplasia in most patients with EBTB. Limited sampling might be the reason for the absence of metaplasia in approximately half of the patients in the present study. Papanicolaou staining showed dense and cyanophilic cytoplasm in the metaplastic non-keratinizing squamous cells.[3] Meanwhile, some keratinizing cells had a bright red color due to the Orange G stain. Atypia in some non-keratinizing squamous cells mainly appeared in the nucleus, presenting as sharply enlarged nuclei (the size of nuclei in the individual cell was almost twice as large as that in surrounding metaplastic cells), nuclear hyperchromasia, and prominent nucleoli. In addition, elongated, polygonal, twisted, and bichromatic cytoplasm in keratinocytes demonstrated atypia and mimicked keratinizing squamous cell carcinoma. Some of the above features have been reported in a previous study;[3] however, features including sharply enlarged nucleus and prominent nucleolus in brushing cytology of EBTB have not been described in detail. An individual cell with enlarged nuclei could not be certainly suspected of malignancy; however, sheets of cells possessing prominent nucleoli may raise more concerns. Although the prominent nucleoli were more consistent with reactive/reparative changes, the same features could also be found in squamous cell carcinoma and adenosquamous carcinoma.[15] In addition, the presence of a larger number of epithelioid cells in the observing field could confuse inexperienced cytologists and result in the consideration of spindle cell neoplasm.
Squamous atypia could not only result in a change of cytomorphology but also disrupt the normal epithelial structure into irregular squamous nests, which are usually surrounded by necrosis with large amounts of MTb. Compared to the only patient who was suspected of malignancy due to the irregular squamous nests in the biopsy, atypia was reported in nine patients in the cytological examination. The interpretations of the differences are as follows. First, the hematoxylin and eosin-stained biopsy specimens displayed the structure of mucosa and stroma in most cases, but the brushing cytology exhibited clusters and scattered cells without continuity. Second, liquid-based cytological examination revealed the morphological characteristics of cells with greater resolution than biopsy pathology, due to better preservation techniques. This allowed for a more detailed analysis of the nucleus.[16] The slightly atypical cellular morphology may easily lead to suspicion in the diagnosis; however, this superiority helps in diagnosing non-small cell lung carcinomas. Third, necrosis showed more interference in cytology than biopsy pathology during diagnosis. Fourth, the detection probability of classical morphological features, such as the Langhans giant cells, was lower in the cytology than in the biopsy pathology.
The diagnosis of TB based on the cytomorphology of bronchial brushing remains challenging for cytologists. However, endobronchial brushing cytology plays an important role in excluding malignant diseases and cannot be abandoned to avoid an uncertain diagnosis of chronic infection.[17,18] Moreover, cytology in a few patients demonstrated Langhans giant cells which were a sign of EBTB and were not encountered in pair biopsy. In general, compared to the dysplastic cells from malignant lesions, squamous reactive atypia can be identified because the degree of atypia and the density of cells are insufficient. Therefore, a good understanding of the typical morphologic features and unusual squamous reactive atypia in exfoliative cytology of EBTB will contribute to an accurate diagnosis.
MTb has not been defined as a biological agent with carcinogenic risk by the International Agency for Research on Cancer.[19] However, a large number of case–control studies demonstrated that chronic inflammation, immune regulation imbalance, and gene mutation caused by TB were related to the occurrence of lung cancer.[20]
In our study, two cases had concurrent lung carcinoma. Therefore, cytomorphology of squamous atypia, regardless of its association with dysplasia, is worthy of in-depth study. In summary, atypia of squamous cells should be noted in the cytopathology report, but cautiousness in the diagnosis of malignancy is necessary. A correct interpretation of bronchial brushing cytology prevents over diagnosis and promotes accurate diagnosis, especially when etiological examinations show negative results. In rare cases, the diagnosis of EBTB should be considered even if cytology and paired biopsy does not exclude dysplasia. Subsequent examination and/or conformation based on clinical response to empirical anti-TB treatment would provide an accurate diagnosis.
Our investigation was mainly limited by the small sample size. Some patients who were lost to follow-up and cases without confirmation from laboratory examinations, despite showing regression or healing of lesions after empirical anti-TB treatment, were not included in the study. This could potentially introduce a selection bias. Furthermore, no high sensitivity and specificity molecular test such as the detection of aberrant methylation changes in the short stature homeobox 2 (SHOX2) and ras association domain family member 1A (RASSF1A) genes were utilized in cases of bronchial TB presenting squamous atypia,[21] which may serve as an effective approach to gain deeper insights into the diagnosis. In addition, Xpert mycobacterium tuberculosis/ rifampicin resistance (Xpert MTB/RIF) and culture of MTb were not performed in our institution due to the lack of the relevant equipment during the study. Last but not least, the negative predictive value of the two pathological examinations could not be calculated because other bronchial inflammatory diseases were not included in the study.
SUMMARYOur study characterized various atypical diagnoses on liquid-based transbronchial brushing cytology at our institution. The accurate identification of epithelioid cells and atypical squamous cells is essential. Although not significant, squamous atypia is a deceptive finding in infectious diseases and should be noted in the report for the appropriate clinical management. However, further examinations, especially those involving invasive methods, should be cautiously performed. In addition, multidisciplinary approaches for diagnosing uncertain cases are necessary.
Comments (0)